Nalbuphine Hydrochloride
NALBUPHINE HYDROCHLORIDE Injection
FULL PRESCRIBING INFORMATION: CONTENTS*
- NALBUPHINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- NALBUPHINE HYDROCHLORIDE INDICATIONS AND USAGE
- NALBUPHINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- NALBUPHINE HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- NALBUPHINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- RL-0188
- RL-1219
- RL-1643
- RL-1220
FULL PRESCRIBING INFORMATION
Ampul
Fliptop Vial
Protect from light.
Rx Only
NALBUPHINE HYDROCHLORIDE DESCRIPTION
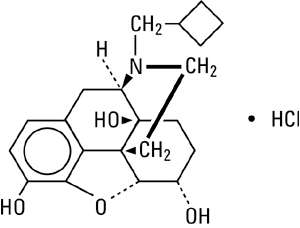
Nalbuphine hydrochloride is a synthetic opioid agonist-antagonist analgesic of the phenanthrene series. It is chemically related to both the widely used opioid antagonist, naloxone, and the potent opioid analgesic, oxymorphone. Chemically nalbuphine hydrochloride is 17-(cyclobutylmethyl)-4,5α-epoxymorphinan-3,6α,14-triol hydrochloride. Nalbuphine hydrochloride molecular weight is 393.91 and is soluble in H2O (35.5 mg/mL at 25ºC) and ethanol (0.8%); insoluble in CHCl3 and ether. Nalbuphine hydrochloride has pKa values of 8.71 and 9.96. The molecular formula is C21H27NO4 • HCl. The structural formula is:
Nalbuphine Hydrochloride Injection is a sterile, nonpyrogenic solution of nalbuphine hydrochloride in water for injection. This product may be administered by subcutaneous, intramuscular or intravenous injection.
Each milliliter (mL) contains nalbuphine hydrochloride 10 mg or 20 mg; sodium citrate, dihydrate 0.47 mg and citric acid, anhydrous 0.63 mg added as buffers and may contain sodium hydroxide and/or hydrochloric acid for pH adjustment; pH 3.7 (3.0 to 4.5). Contains sodium chloride for tonicity adjustment.
Multiple-dose vials contain 1.8 mg/mL methylparaben and 0.2 mg/mL propylparaben added as preservatives. Single-dose products contain no bacteriostat or antimicrobial agent and unused portions must be discarded.
CLINICAL PHARMACOLOGY
Nalbuphine hydrochloride is a potent analgesic. Its analgesic potency is essentially equivalent to that of morphine on a milligram basis. Receptor studies show that nalbuphine hydrochloride binds to mu, kappa, and delta receptors, but not to sigma receptors. Nalbuphine hydrochloride is primarily a kappa agonist/partial mu antagonist analgesic.
The onset of action of nalbuphine hydrochloride occurs within 2 to 3 minutes after intravenous administration, and in less than 15 minutes following subcutaneous or intramuscular injection. The plasma half-life of nalbuphine is 5 hours, and in clinical studies, the duration of analgesic activity has been reported to range from 3 to 6 hours.
The opioid antagonist activity of nalbuphine is one-fourth as potent as nalorphine and 10 times that of pentazocine.
Nalbuphine hydrochloride may produce the same degree of respiratory depression as equianalgesic doses of morphine. However, nalbuphine hydrochloride exhibits a ceiling effect such that increases in dose greater than 30 mg do not produce further respiratory depression in the absence of other CNS active medications affecting respiration.
Nalbuphine hydrochloride by itself has potent opioid antagonist activity at doses equal to or lower than its analgesic dose. When administered following or concurrent with mu agonist opioid analgesics (e.g., morphine, oxymorphone, fentanyl), nalbuphine hydrochloride may partially reverse or block opioid-induced respiratory depression from the mu agonist analgesic. Nalbuphine hydrochloride may precipitate withdrawal in patients dependent on opioid drugs. Nalbuphine hydrochloride should be used with caution in patients who have been receiving mu opioid analgesics on a regular basis.
NALBUPHINE HYDROCHLORIDE INDICATIONS AND USAGE
Nalbuphine hydrochloride is indicated for the relief of moderate to severe pain. Nalbuphine hydrochloride can also be used as a supplement to balanced anesthesia, for preoperative and postoperative analgesia, and for obstetrical analgesia during labor and delivery.
NALBUPHINE HYDROCHLORIDE CONTRAINDICATIONS
Nalbuphine hydrochloride injection should not be administered to patients who are hypersensitive to nalbuphine hydrochloride, or to any of the other ingredients in nalbuphine hydrochloride injection.
WARNINGS
Nalbuphine hydrochloride injection should be administered as a supplement to general anesthesia only by persons specifically trained in the use of intravenous anesthetics and management of respiratory effects of potent opioids.
Naloxone hydrochloride injection, resuscitative and intubation equipment and oxygen should be readily available.
Drug Abuse
Caution should be observed in prescribing nalbuphine for emotionally unstable patients, or for individuals with a history of opioid abuse. Such patients should be closely supervised when long term therapy is contemplated (see DRUG ABUSE AND DEPENDENCE ).
Use in Ambulatory Patients
Nalbuphine may impair the mental or physical abilities required for the performance of potentially dangerous tasks such as driving a car or operating machinery. Therefore, nalbuphine hydrochloride injection should be administered with caution to ambulatory patients who should be warned to avoid such hazards.
Use in Emergency Procedures
Maintain patient under observation until recovered from nalbuphine effects that would affect driving or other potentially dangerous tasks.
Use in Pregnancy (Other Than Labor)
Severe fetal bradycardia has been reported when nalbuphine is administered during labor. Naloxone may reverse these effects. Although there are no reports of fetal bradycardia earlier in pregnancy, it is possible that this may occur. This drug should be used in pregnancy only if clearly needed, if the potential benefit outweighs the risk to the fetus, and if appropriate measures such as fetal monitoring are taken to detect and manage any potential adverse effect on the fetus.
Use During Labor and Delivery
The placental transfer of nalbuphine is high, rapid, and variable with a maternal to fetal ratio ranging from 1:0.37 to 1:6. Fetal and neonatal adverse effects that have been reported following the administration of nalbuphine to the mother during labor include fetal bradycardia, respiratory depression at birth, apnea, cyanosis, and hypotonia. Some of these events have been life-threatening. Maternal administration of naloxone during labor has normalized these effects in some cases. Severe and prolonged fetal bradycardia has been reported. Permanent neurological damage attributed to fetal bradycardia has occurred. A sinusoidal fetal heart rate pattern associated with the use of nalbuphine has also been reported. Nalbuphine should be used during labor and delivery only if clearly indicated and only if the potential benefit outweighs the risk to the infant. Newborns should be monitored for respiratory depression, apnea, bradycardia and arrhythmias if nalbuphine has been used.
Head Injury and Increased Intracranial Pressure
The possible respiratory depressant effects and the potential of potent analgesics to elevate cerebrospinal fluid pressure (resulting from vasodilation following CO2 retention) may be markedly exaggerated in the presence of head injury, intracranial lesions or a pre-existing increase in intracranial pressure. Furthermore, potent analgesics can produce effects which may obscure the clinical course of patients with head injuries. Therefore, nalbuphine hydrochloride injection should be used in these circumstances only when essential, and then should be administered with extreme caution.
Interaction With Other Central Nervous System Depressants
Although nalbuphine possesses opioid antagonist activity, there is evidence that in nondependent patients it will not antagonize an opioid analgesic administered just before, concurrently, or just after an injection of nalbuphine hydrochloride. Therefore, patients receiving an opioid analgesic, general anesthetics, phenothiazines, or other tranquilizers, sedatives, hypnotics, or other CNS depressants (including alcohol) concomitantly with nalbuphine may exhibit an additive effect. When such combined therapy is contemplated, the dose of one or both agents should be reduced.
PRECAUTIONS
General
Impaired Respiration: At the usual adult dose of 10 mg/70 kg, nalbuphine hydrochloride causes some respiratory depression approximately equal to that produced by equal doses of morphine. However, in contrast to morphine, respiratory depression is not appreciably increased with higher doses of nalbuphine. Respiratory depression induced by nalbuphine can be reversed by naloxone hydrochloride when indicated. Nalbuphine hydrochloride injection should be administered with caution at low doses to patients with impaired respiration (e.g., from other medication, uremia, bronchial asthma, severe infection, cyanosis or respiratory obstructions).
Impaired Renal or Hepatic Function: Because nalbuphine is metabolized in the liver and excreted by the kidneys, nalbuphine hydrochloride should be used with caution in patients with renal or liver dysfunction and administered in reduced amounts.
Myocardial Infarction: As with all potent analgesics, nalbuphine hydrochloride should be used with caution in patients with myocardial infarction who have nausea or vomiting.
Biliary Tract Surgery: As with all opioid analgesics, nalbuphine hydrochloride should be used with caution in patients about to undergo surgery of the biliary tract since it may cause spasm of the sphincter of Oddi.
Cardiovascular System: During evaluation of nalbuphine hydrochloride injection, in anesthesia, a higher incidence of bradycardia has been reported in patients who did not receive atropine pre-operatively.
Information for Patients
Patients should be advised of the following information:
-
Nalbuphine is associated with sedation and may impair mental and physical abilities required for the performance of potentially dangerous tasks such as driving a car or operating machinery.
-
Nalbuphine is to be used as prescribed by a physician. Dose or frequency should not be increased without first consulting with a physician since nalbuphine may cause psychological or physical dependence.
-
The use of nalbuphine with other opioids can cause signs and symptoms of withdrawal.
-
Abrupt discontinuation of nalbuphine after prolonged usage may cause signs and symptoms of withdrawal.
Laboratory Tests
Nalbuphine hydrochloride may interfere with enzymatic methods for the detection of opioids depending on the specificity/sensitivity of the test. Consult the test manufacturer for specific details.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: Long term carcinogenicity studies were performed in rats (24 months) and mice (19 months) by oral administration at doses up to 200 mg/kg (1180 mg/m2) and 200 mg/kg (600 mg/m2) per day, respectively. There was no evidence of an increase in tumors in either species related to nalbuphine hydrochloride administration. The maximum recommend human dose (MRHD) in a day is 160 mg subcutaneously, intramuscularly or intravenously, or approximately 100 mg/m2/day for a 60 kg subject.
Mutagenesis: Nalbuphine hydrochloride did not have mutagenic activity in the AMES test with four bacterial strains, in the Chinese Hamster Ovary HGPRT assays or in the Sister Chromatids Exchange Assay. However, nalbuphine hydrochloride induced an increased frequency of mutation in the mouse lymphoma assay. Clastogenic activity was not observed in the mouse micronucleus test of the cytogenicity bone marrow assay in rats.
Impairment of Fertility: A reproduction study was performed in male and female rats at subcutaneous doses up to 56 mg/kg/day or 330 mg/m2/day. Nalbuphine hydrochloride did not affect either male or female fertility rats.
Usage in Pregnancy
Teratogenic Effects:
Pregnancy Category B: Reproduction studies have been performed in rats by subcutaneous administration of nalbuphine up to 100 mg/kg/day, or 590 mg/m2/day which is approximately 6 times the MRHD, and in rabbits by intravenous administration of nalbuphine up to 32 mg/kg/day, or 378 mg/m2/day which is approximately 4 times the MRHD. The results did not reveal evidence of developmental toxicity, including teratogenicity, or harm to the fetus. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Non-teratogenenic Effects:
Neonatal body weight and survival rates were reduced at birth and during lactation when nalbuphine was subcutaneously administered to female and male rats prior to mating and throughout gestation and lactation or to pregnant rats during the last third of gestation and throughout lactation at doses approximately 4 times the maximum recommended human dose.
Use During Labor and Delivery
See WARNINGS .
Nursing Mothers
Limited data suggest that nalbuphine hydrochloride is excreted in maternal milk but only in a small amount (less than 1% of the administered dose) and with a clinically insignificant effect. Caution should be exercised when nalbuphine hydrochloride is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 18 years have not been established.
NALBUPHINE HYDROCHLORIDE ADVERSE REACTIONS
The most frequent adverse reaction in 1066 patients treated with nalbuphine hydrochloride injection was sedation 381 (36%). Less frequent reactions were: sweaty/clammy 99 (9%), nausea/vomiting 68 (6%), dizziness/vertigo 58 (5%), dry mouth 44 (4%), and headache 27 (3%).
Other adverse reactions which occurred (reported incidence of 1% or less) were:
CNS Effects: Nervousness, depression, restlessness, crying, euphoria, floating, hostility, unusual dreams, confusion, faintness, hallucinations, dysphoria, feeling of heaviness, numbness, tingling, unreality. The incidence of psychotomimetic effects, such as unreality, depersonalization, delusions, dysphoria and hallucinations has been shown to be less than that which occurs with pentazocine.
Cardiovascular: Hypertension, hypotension, bradycardia, tachycardia.
Gastrointestinal: Cramps, dyspepsia, bitter taste.
Respiratory: Depression, dyspnea, asthma.
Dermatologic: Itching, burning, urticaria.
Miscellaneous: Speech difficulty, urinary urgency, blurred vision, flushing and warmth.
Allergic Reactions: Anaphylactic/anaphylactoid and other serious hypersensitivity reactions have been reported following the use of nalbuphine and may require immediate, supportive medical treatment. These reactions may include shock, respiratory distress, respiratory arrest, bradycardia, cardiac arrest, hypotension, or laryngeal edema. Some of these allergic reactions may be life-threatening. Other allergic-type reactions reported include stridor, bronchospasm, wheezing, edema, rash, pruritus, nausea, vomiting, diaphoresis, weakness, and shakiness.
Events Observed during Post-marketing Surveillance of Nalbuphine Hydrochloride Injection
Due to the nature and limitations of spontaneous reporting, causality has not been established for the following adverse events received for nalbuphine hydrochloride injection: abdominal pain, pyrexia, depressed level or loss of consciousness, somnolence, tremor, anxiety, pulmonary edema, agitation, seizures, and injection site reactions such as pain, swelling, redness, burning, and hot sensations. Death has been reported from severe allergic reactions to nalbuphine hydrochloride treatment. Fetal death has been reported where mothers received nalbuphine hydrochloride during labor and delivery.
DRUG ABUSE AND DEPENDENCE
There have been reports of abuse and dependence associated with nalbuphine hydrochloride among health care providers, patients and bodybuilders. There have been reported instances of psychological and physical dependence and tolerance in patients abusing nalbuphine hydrochloride. Individuals with a prior history of opioid or other substance abuse or dependence may be at greater risk in responding to reinforcing properties of nalbuphine hydrochloride.
Abrupt discontinuation of nalbuphine hydrochloride following prolonged use has been followed by symptoms of opioid withdrawal, i.e., abdominal cramps, nausea and vomiting, rhinorrhea, lacrimation, restlessness, anxiety, elevated temperature and piloerection.
OVERDOSAGE
The immediate intravenous administration of an opiate antagonist such as naloxone or nalmefene is a specific antidote. Oxygen, intravenous fluids, vasopressors and other supportive measures should be used as indicated.
The administration of single doses of 72 mg of nalbuphine hydrochloride subcutaneously to eight normal subjects has been reported to have resulted primarily in symptoms of sleepiness and mild dysphoria.
NALBUPHINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
The usual recommended adult dose is 10 mg for a 70 kg individual administered subcutaneously, intramuscularly, or intravenously; this dose may be repeated every 3 to 6 hours as necessary. Dosage should be adjusted according to the severity of the pain, physical status of the patient, and other medications which the patient may be receiving (see Interaction With Other Central Nervous System Depressants under WARNINGS ). In nontolerant individuals, the recommended single maximum dose is 20 mg with a maximum total daily dose of 160 mg.
The use of nalbuphine hydrochloride injection as a supplement to balanced anesthesia requires larger doses than those recommended for analgesia. Induction doses of nalbuphine hydrochloride range from 0.3 mg/kg to 3 mg/kg intravenously to be administered over a 10 to 15 minute period with maintenance doses of 0.25 to 0.5 mg/kg in single intravenous administrations as required. The use of nalbuphine hydrochloride injection may be followed by respiratory depression which can be reversed with the opioid antagonist naloxone hydrochloride.
Patients Dependent on Opioids: Patients who have been taking opioids chronically may experience withdrawal symptoms upon the administration of nalbuphine hydrochloride injection. If unduly troublesome, opioid withdrawal symptoms can be controlled by the slow intravenous administration of small increments of morphine, until relief occurs. If the previous analgesic was morphine, meperidine, codeine, or other opioid with similar duration of activity, one-fourth of the anticipated dose of nalbuphine hydrochloride can be administered initially and the patient observed for signs of withdrawal, i.e., abdominal cramps, nausea and vomiting, lacrimation, rhinorrhea, anxiety, restlessness, elevation of temperature or piloerection. If untoward symptoms do not occur, progressively larger doses may be tried at appropriate intervals until the desired level of analgesia is obtained with nalbuphine hydrochloride.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
HOW SUPPLIED
Nalbuphine Hydrochloride Injection is supplied as follows:
|
NDC No. |
Container |
Size (mL) |
mg/mL |
Total mg |
|
0409–1463–01 |
Ampul |
1 |
10 |
10 |
|
0409–1465–01 |
Ampul |
1 |
20 |
20 |
|
0409–1464–01 |
Fliptop Vial (multiple-dose) |
10 |
10 |
100 |
|
0409–1467–01 |
Fliptop Vial (multiple-dose) |
10 |
20 |
200 |
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
Protect from light. Store in carton until contents have been used.
Revised: August, 2007
Printed in USA EN-1571
Hospira, Inc., Lake Forest, IL 60045 USA
RL-0188

RL-1219

RL-1643

RL-1220

Nalbuphine HydrochlorideNALBUPHINE HYDROCHLORIDE INJECTION, SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Nalbuphine HydrochlorideNALBUPHINE HYDROCHLORIDE INJECTION, SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Nalbuphine HydrochlorideNALBUPHINE HYDROCHLORIDE INJECTION, SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Nalbuphine HydrochlorideNALBUPHINE HYDROCHLORIDE INJECTION, SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||