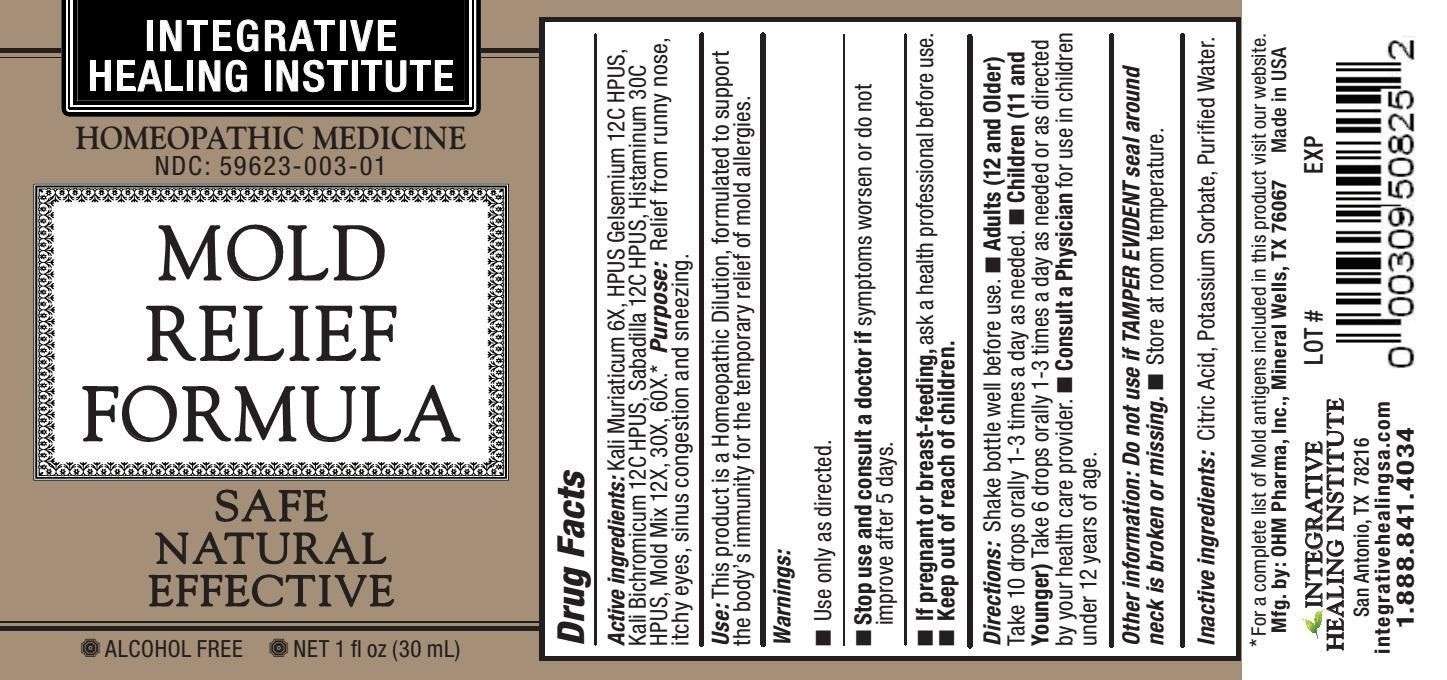
Mold Relief Formula
Integrative Healing Institute, LLC
OHM PHARMA INC.
MOLD RELIEF FORMULA
FULL PRESCRIBING INFORMATION
Active ingredient
Active ingredients : Kali Muriaticum 6X HPUS, Gelsemium 12C HPUS, Kali Bichromicum 12C HPUS, Sabadilla 12C HPUS, Histaminum 30C HPUS, Mold Mix 12X, 30X, 60X.*
Purpose
Purpose: Relief from runny nose, itchy eyes, sinus congestion and sneezing.
Uses
Use: This product is a Homeopathic Dilution, formulated to support the body's immunity for the temporary relief of mold allergies.
W arnings:
- Use only as directed.
- Stop use and consult a doctor if symptoms worsen or do not improve after 5 days.
- If pregnant or breast-feeding, ask a health professional before use.
- Keep out of reach of children.
Directions: Shake bottle well before use.
- Adults (12 and Older) Take 10 drops orally 1-3 times a day as needed.
- Children (11 and Younger) Take 6 drops orally 1-3 times a day as needed or as directed by your health care provider.
- Consult a Physician for use in children under 12 years of age.
Other information: Do not use if TAMPER EVIDENT seal around neck is broken or missing.
- Store at room temperature.
Inactive ingredients: Citric Acid, Potassium Sorbate, Purified Water.
* For a complete list of Mold antigens included in this product visit our website.
Mfg. by: OHM Pharma, Inc., Mineral Wells, TX 76067
Made in USA
INTEGRATIVE HEALING INSTITUTE
San Antonio, TX 78216
integrativehealingsa.com
1.888.841.4034
INTEGRATIVE HEALING INSTITUTE
HOMEOPATHIC MEDICINE
- NDC: 59623-003-01
MOLD RELIEF FORMULA
- SAFE
- NATURAL
- EFFECTIVE
- ALCOHOL FREE
- NET 1fl oz (30 mL)
Mold Relief FormulaKali Muriaticum, Gelsemium, Kali Bichromicum, Sabadilla, Histaminum, Mold Mix. LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||