Methotrexate Sodium
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- METHOTREXATE SODIUM DESCRIPTION
- INACTIVE INGREDIENT
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- INDICATIONS & USAGE
- METHOTREXATE SODIUM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- METHOTREXATE SODIUM ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- HOW SUPPLIED
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
PRECAUTIONS
CONTRAINDICATIONS
Drug Interactions
Organ System Toxicity
Organ System Toxicity
METHOTREXATE SODIUM DESCRIPTION
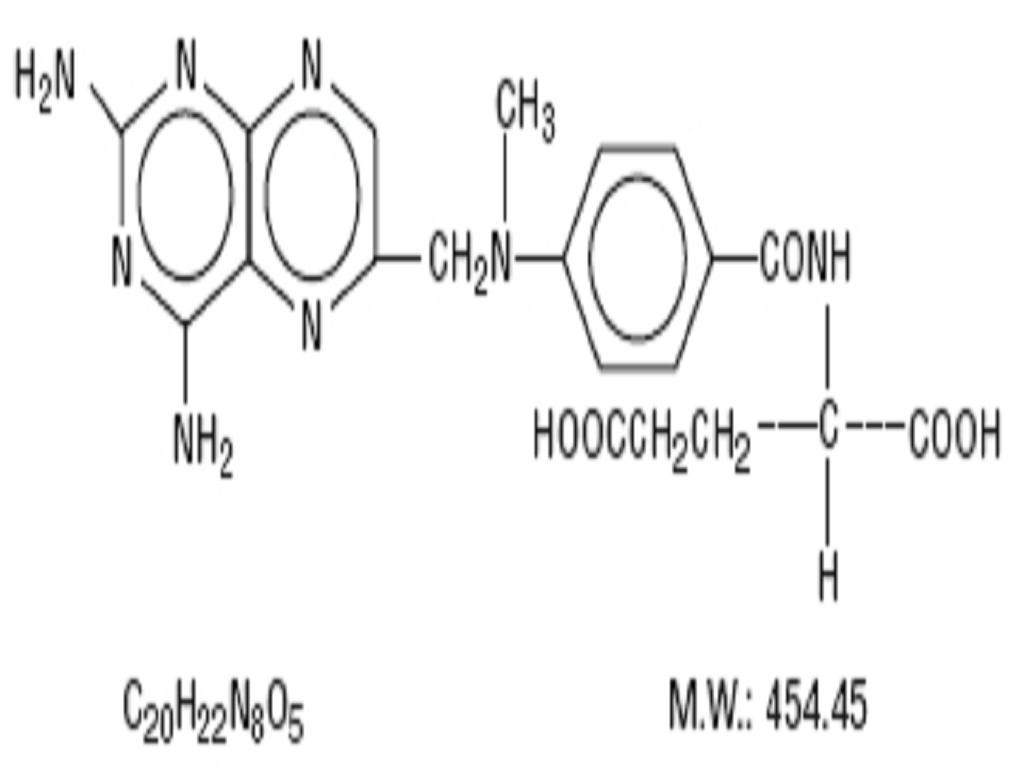
INACTIVE INGREDIENT
CLINICAL PHARMACOLOGY
PHARMACOKINETICS
INDICATIONS & USAGE
Neoplastic DiseasesPsoriasis
Rheumatoid Arthritis including Polyarticular-Course Juvenile Rheumatoid Arthritis
Drug Interactions
METHOTREXATE SODIUM CONTRAINDICATIONS
PRECAUTIONSBoxed WARNINGS.)Because of the potential for serious adverse reactions from methotrexate in breast fed infants, it is contraindicated in nursing mothers.
Patients with psoriasis or rheumatoid arthritis with alcoholism, alcoholic liver disease or other chronic liver disease should not receive methotrexate.
Patients with psoriasis or rheumatoid arthritis who have overt or laboratory evidence of immunodeficiency syndromes should not receive methotrexate.
Patients with psoriasis or rheumatoid arthritis who have preexisting blood dyscrasias, such as bone marrow hypoplasia, leukopenia, thrombocytopenia or significant anemia, should not receive methotrexate.
Patients with a known hypersensitivity to methotrexate should not receive the drug.
WARNINGS
SEE BOXED WARNINGS.PRECAUTIONS
GeneralBoxed WARNINGSOVERDOSAGE
INFORMATION FOR PATIENTS
LABORATORY TESTS
Organ System Toxicity
DRUG INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
CONTRAINDICATIONSNURSING MOTHERS
CONTRAINDICATIONSPEDIATRIC USE
CLINICAL PHARMACOLOGYADVERSE REACTIONSDOSAGE AND ADMINISTRATION
GERIATRIC USE
Drug InteractionsBoxed WARNINGSADVERSE REACTIONSOrgan System Toxicity
METHOTREXATE SODIUM ADVERSE REACTIONS
Adverse Reactions in Double-Blind Rheumatoid Arthritis Studies
PRECAUTIONS
PRECAUTIONS
Adverse Reactions in Psoriasis
Adverse Reactions in JRA Studies
OVERDOSAGE
DOSAGE & ADMINISTRATION
Neoplastic DiseasesPsoriasis, Rheumatoid Arthritis, and Juvenile Rheumatoid Arthritis
Information for PatientsPRECAUTIONSPRECAUTIONSCONTRAINDICATIONS
ADVERSE REACTIONS
STORAGE AND HANDLING
HANDLING AND DISPOSAL
HOW SUPPLIED
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Methotrexate SodiumMethotrexate Sodium TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!