MENOMUNE - A/C/Y/W-135 COMBINED
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Menomune – A/C/Y/W-135 vaccine safely and effectively. See full prescribing information for Menomune – A/C/Y/W-135 vaccine. Menomune – A/C/Y/W-135, Meningococcal Polysaccharide Vaccine, Groups A, C, Y and W-135 Combined Solution for subcutaneous injection Initial U.S. Approval: 1981INDICATIONS AND USAGE Menomune – A/C/Y/W-135 vaccine is a vaccine indicated for active immunization against invasive meningococcal disease caused by Neisseria meningitidis serogroups A, C, Y, and W-135. Menomune – A/C/Y/W-135 vaccine is approved for use in persons 2 years of age and older. Menomune – A/C/Y/W-135 vaccine does not prevent N meningitidis serogroup B disease. (1) DOSAGE AND ADMINISTRATION Single 0.5 mL subcutaneous injection. (2) DOSAGE FORMS AND STRENGTHS Menomune – A/C/Y/W-135 vaccine is supplied as a single dose or multidose (10 dose) vial of lyophilized vaccine, with corresponding single dose or multidose vial of diluent. After reconstitution of the lyophilized vaccine with the diluent, each dose consists of a 0.5 mL solution for injection. (3) CONTRAINDICATIONS Do not administer to anyone with a history of a severe allergic reaction (e.g., anaphylaxis) to Menomune – A/C/Y/W-135 vaccine or any component of the vaccine. (4) WARNINGS AND PRECAUTIONS The stoppers to the vials of lyophilized vaccine and diluent contain dry natural latex rubber that may cause allergic reactions. (5.1) Side Effects Common (>10%) adverse events in children 2 to 10 years of age were injection site pain, drowsiness, irritability, and diarrhea. (6) Common (>10%) adverse events in persons 11 to 55 years of age were pain, redness, and induration at the injection site, headache, fatigue, malaise, arthralgia, and diarrhea. (6) To report SUSPECTED ADVERSE REACTIONS, contact Sanofi Pasteur Inc. at 1-800-822-2463 (1-800-VACCINE) or VAERS at 1-800-822-7967 or http://vaers.hhs.gov. DRUG INTERACTIONS Do not mix Menomune – A/C/Y/W-135 vaccine with other vaccines in the same syringe or vial. (7) Immunosuppressive therapies may reduce the immune response to Menomune – A/C/Y/W-135 vaccine. (7) No safety and immunogenicity data are available on the concomitant administration of Menomune – A/C/Y/W-135 vaccine with other US licensed vaccines. (7) USE IN SPECIFIC POPULATIONS Pregnancy: No human or animal data. Use only if clearly needed. (8.1) Geriatrics: Not known whether persons aged 65 years and older respond differently from younger persons. (8.5)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1. MENOMUNE - A/C/Y/W-135 COMBINED INDICATIONS AND USAGE
- 2. MENOMUNE - A/C/Y/W-135 COMBINED DOSAGE AND ADMINISTRATION
- 3. DOSAGE FORMS AND STRENGTHS
- 4. MENOMUNE - A/C/Y/W-135 COMBINED CONTRAINDICATIONS
- 5. WARNINGS AND PRECAUTIONS
- 6. MENOMUNE - A/C/Y/W-135 COMBINED ADVERSE REACTIONS
- 7. DRUG INTERACTIONS
- 8. USE IN SPECIFIC POPULATIONS
- 11. MENOMUNE - A/C/Y/W-135 COMBINED DESCRIPTION
- 12. CLINICAL PHARMACOLOGY
- 13. NON-CLINICAL TOXICOLOGY
- 14. CLINICAL STUDIES
- 15. REFERENCES
- 16. HOW SUPPLIED/STORAGE AND HANDLING
- 17. PATIENT COUNSELING INFORMATION
FULL PRESCRIBING INFORMATION
1. INDICATIONS AND USAGE
Menomune® – A/C/Y/W-135, Meningococcal Polysaccharide Vaccine, Groups A, C, Y and W-135 Combined, is indicated for active immunization for the prevention of invasive meningococcal disease caused by Neisseria meningitidis serogroups A, C, Y, and W-135.
Menomune – A/C/Y/W-135 vaccine is approved for use in persons 2 years of age and older.
Menomune – A/C/Y/W-135 vaccine does not prevent N meningitidis serogroup B disease.
2. DOSAGE AND ADMINISTRATION
2.1. Administration
The package contains a vial of lyophilized vaccine and a vial of diluent. The lyophilized vaccine should be a white or off-white color to a light beige color. The diluent used for reconstitution is a clear liquid.
After removing the "flip-off" caps, cleanse the vaccine and diluent vial stoppers with a suitable germicide. Do not remove the vial stoppers or metal seals holding them in place. Using a suitable sterile needle and syringe and aseptic technique, withdraw the supplied diluent (0.6 mL for single dose presentation and 6.0 mL for multidose presentation) (Refer to Figure 1) and inject into the vial containing the lyophilized vaccine (Refer to Figure 2). Swirl the vial until the vaccine is thoroughly dissolved (Refer to Figure 3). When reconstituted, the vaccine should be a clear, colorless liquid.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If either of these conditions exists, the vaccine should not be administered.
Using a suitable sterile needle and syringe and aseptic technique, withdraw (Refer to Figure 4) and administer a 0.5 mL dose of Menomune – A/C/Y/W-135 vaccine by subcutaneous injection. Use a separate sterile needle and syringe and aseptic technique for each dose withdrawn from the multidose vial.
Do not administer this product intravenously or intramuscularly.
The preferred site of administration is the deltoid region.
Menomune vaccine: Instructions for reconstitution
Figure 1
For the single-dose presentation, withdraw 0.6 mL of the supplied diluent; for the multidose presentation, withdraw 6.0 mL.
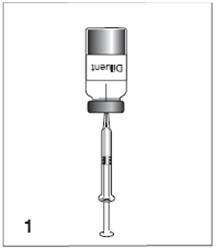
Figure 2
Insert the syringe needle through the stopper of the vial of lyophilized Menomune vaccine component and inject the diluent into the vial.
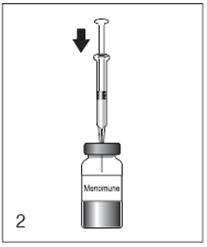
Figure 3
Swirl the vial until the lyophilized vaccine component is thoroughly dissolved.
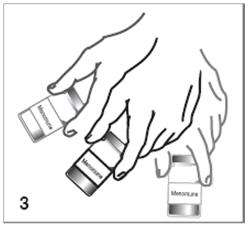
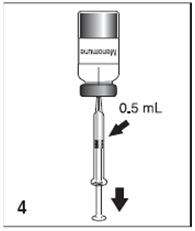
Figure 4
After reconstitution, withdraw 0.5 mL of Menomune vaccine and administer subcutaneously. Use the single-dose presentation immediately after reconstitution. The multidose presentation may be used for up to 35 days after reconstitution if stored at 2° to 8°C.
The vaccine should not be combined through reconstitution or mixed with any other vaccine.
Vaccine supplied in single dose vials should be used immediately after reconstitution. Vaccine supplied in multidose vials may be used for up to 35 days after reconstitution if stored at 2° to 8°C (35° to 46°F). [See How Supplied/Storage and Handling (16.2) .]
2.2. Primary Immunization
Primary immunization with Menomune – A/C/Y/W-135 vaccine consists of a single 0.5 mL dose administered subcutaneously.
The ACIP (Advisory Committee on Immunization Practices) has specific recommendations for use of meningococcal vaccines. (1) (2) (3)
2.3. Revaccination
The ACIP has recommendations for revaccination against meningococcal disease for persons at high risk who were previously vaccinated with Menomune – A/C/Y/W-135 vaccine. (1) (3) If Menomune – A/C/Y/W-135 vaccine is used for revaccination, the dose is 0.5 mL administered subcutaneously.
3. DOSAGE FORMS AND STRENGTHS
Menomune – A/C/Y/W-135 vaccine is supplied as a lyophilized vaccine, in a single dose or a multidose (10 dose) vial, with corresponding single dose or multidose vial of diluent. The lyophilized vaccine is reconstituted with the diluent [see Dosage and Administration (2.1) ]. After reconstitution, each dose consists of a 0.5 mL solution for injection. See Description (11) for the complete listing of ingredients.
4. CONTRAINDICATIONS
4.1. Hypersensitivity
Do not administer to anyone with a history of a severe allergic reaction (e.g., anaphylaxis) to Menomune – A/C/Y/W-135 vaccine or any component of the vaccine [see Description (11) ].
5. WARNINGS AND PRECAUTIONS
5.1. Latex
The stoppers to the vials of lyophilized vaccine and diluent contain dry natural latex rubber that may cause allergic reactions in latex sensitive persons.
5.2. Management of Acute Allergic Reactions
Appropriate medical treatment must be available to manage possible anaphylactic reactions following administration of the vaccine.
5.3. Moderate or Severe Acute Illness
To avoid diagnostic confusion between manifestations of an acute illness and possible vaccine adverse effects, vaccination with Menomune – A/C/Y/W-135 vaccine should be postponed in persons with moderate or severe acute illness. (4)
5.4. Limitations of Vaccine Effectiveness
Menomune – A/C/Y/W-135 vaccine may not protect all recipients.
5.5. Altered Immunocompetence
Persons who are immunosuppressed, including persons receiving immunosuppressive therapy, may have a diminished immune response to Menomune – A/C/Y/W-135 vaccine [see Drug Interactions (7) ].
6. ADVERSE REACTIONS
6.1. Data from Clinical Studies
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in clinical practice.
In three clinical trials primarily designed to assess the safety and immunogenicity of another vaccine, Menactra® [Meningococcal (Groups A, C, Y and W-135) Polysaccharide Diphtheria Toxoid Conjugate Vaccine], participants were randomized to receive Menactra or Menomune – A/C/Y/W-135 vaccine, which was used as a control vaccine. In these three trials, 1519 children 2-10 years of age, 972 persons 11-18 years of age, and 1170 adults 18-55 years of age, respectively, received a dose of Menomune – A/C/Y/W-135 vaccine. Overall, of the children 2-10 years of age who received Menactra or Menomune – A/C/Y/W-135 vaccine, 68% were enrolled at US sites and 32% were enrolled at a Chilean site. The median ages of US and Chilean children were 6 and 5 years, respectively; overall, 50.5% were males and 92.0% were Caucasian. Among participants 11-55 years of age who received Menactra or Menomune – A/C/Y/W-135 vaccine, all were enrolled at US sites; 54.8% were female; 87.7% were Caucasian.
Solicited local and systemic reactions were monitored daily for 7 days post-vaccination using a diary card. Information on serious adverse events was collected at interim clinic visits and by telephone interview conducted 6 months post-vaccination. At least 94% of participants from the three studies completed the 6-month follow-up.
Serious Adverse Events
Across the three studies, serious adverse events within 6-months following Menomune – A/C/Y/W-135 vaccine were reported in 0.7% of 1519 children 2-10 years of age, 0.6% of 972 persons 11-18 years of age, and 1.7% of 1170 persons 18-55 years of age.
Solicited Adverse Events
The most commonly reported solicited adverse events in US children 2-10 years of age were injection site pain, irritability, and diarrhea. (Table 1)
The most commonly reported solicited adverse events in adolescents, ages 11-18 years, and adults, ages 18-55 years, were injection site pain, headache, and fatigue. (Table 2)
| N=1019-1027 |
|||
|---|---|---|---|
| Event | Any | Moderate | Severe |
| % | % | % | |
| General disorders and administration site conditions | |||
| Injection site reaction | |||
| Pain |
26.1 | 2.5 | 0.0 |
Redness |
7.9 | 0.5 | 0.0 |
Induration |
4.2 | 0.6 | 0.0 |
Swelling |
2.8 | 0.3 | 0.0 |
| Systemic events | |||
| Fever |
5.2 | 1.7 | 0.2 |
| Gastrointestinal disorders | |||
| Anorexia |
8.7 | 1.3 | 0.8 |
| Vomiting |
2.7 | 0.7 | 0.6 |
| Diarrhea |
11.8 | 2.5 | 0.3 |
| Nervous system disorders | |||
| Drowsiness |
11.2 | 2.5 | 0.5 |
| Irritability |
12.2 | 2.6 | 0.6 |
Seizures |
0.0 | N/A | N/A |
| Musculoskeletal and connective tissue disorders | |||
| Arthralgia |
5.3 | 0.7 | 0.0 |
| Skin and subcutaneous disorders | |||
Rash |
3.0 | N/A | N/A |
| Study 1 N  |
Study 2 N  |
|||||
|---|---|---|---|---|---|---|
| Participants 11-18 years of age | Participants 18-55 years of age | |||||
| Event | Any | Moderate | Severe | Any | Moderate | Severe |
| General disorders and administration site conditions | ||||||
| Injection site reaction | ||||||
| Pain |
28.7 | 2.6 | 0.0 | 48.1 | 3.3 | 0.1 |
Redness |
5.7 | 0.4 | 0.0 | 16.0 | 1.9 | 0.1 |
Induration |
5.2 | 0.5 | 0.0 | 11.0 | 1.0 | 0.0 |
Swelling |
3.6 | 0.3 | 0.0 | 7.6 | 0.7 | 0.0 |
| Systemic events | ||||||
Fatigue |
25.1 | 6.2 | 0.2 | 32.3 | 6.6 | 0.4 |
Malaise |
16.8 | 3.4 | 0.4 | 22.3 | 4.7 | 0.9 |
Chills |
3.5 | 0.4 | 0.1 | 5.6 | 1.0 | 0.0 |
| Fever |
3.0 | 0.3 | 0.1 | 0.5 | 0.1 | 0.0 |
| Gastrointestinal disorders | ||||||
| Diarrhea |
10.2 | 1.3 | 0.0 | 14.0 | 2.9 | 0.3 |
| Anorexia |
7.7 | 1.1 | 0.2 | 9.9 | 1.6 | 0.4 |
| Vomiting |
1.4 | 0.5 | 0.3 | 1.5 | 0.2 | 0.4 |
| Nervous system disorders | ||||||
| Headache |
29.3 | 6.5 | 0.4 | 41.8 | 8.9 | 0.9 |
Seizure |
0.0 | N/A | N/A | 0.0 | N/A | N/A |
| Musculoskeletal and connective tissue disorders | ||||||
Arthralgia |
10.2 | 2.1 | 0.1 | 16.0 | 2.6 | 0.1 |
| Skin and subcutaneous disorders | ||||||
Rash |
1.4 | N/A | N/A | 0.8 | N/A | N/A |
6.2. Data from Post-Marketing Experience
The following adverse events have been spontaneously reported during post-approval use of Menomune – A/C/Y/W-135 vaccine since 1993 through November 2008. Because these events were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or to establish a causal relationship to Menomune – A/C/Y/W-135 vaccine exposure.
The following adverse events were included based on severity, frequency of reporting or the strength of causal association to Menomune – A/C/Y/W-135 vaccine.
- Immune System Disorders
-
-
- Nervous System Disorders
-
-
- Gastrointestinal Disorders
-
-
- Musculoskeletal and Connective Tissue Disorders
-
-
- General Disorders and Administration Site Conditions
-
-
7. DRUG INTERACTIONS
Separate syringes and injection sites must be used in case of concomitant administration.
Do not mix Menomune – A/C/Y/W-135 vaccine with other vaccines in the same syringe or vial.
Immunosuppressive therapies may reduce the immune response to Menomune – A/C/Y/W-135 vaccine.
No safety and immunogenicity data are available on the concomitant administration of Menomune – A/C/Y/W-135 vaccine with other US licensed vaccines.
8. USE IN SPECIFIC POPULATIONS
8.1. Pregnancy
Pregnancy Category C. Animal reproduction studies have not been conducted with Menomune – A/C/Y/W-135 vaccine. It is also not known whether Menomune – A/C/Y/W-135 vaccine can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Menomune – A/C/Y/W-135 vaccine should be given to a pregnant woman only if clearly needed.
8.3. Nursing Mothers
It is not known whether Menomune – A/C/Y/W-135 vaccine is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Menomune – A/C/Y/W-135 vaccine is administered to a nursing woman.
8.4. Pediatric Use
Safety and effectiveness of Menomune – A/C/Y/W-135 vaccine in children below the age of 2 years have not been established.
During a meningococcal serogroup A epidemic in sub-Saharan Africa, children 3 months to 16 years of age were vaccinated with a high molecular weight serogroup A/C meningococcal polysaccharide vaccine. In case-control studies, after 1 year of observation, vaccine efficacy against meningococcal serogroup A disease was estimated to be 87% [90% Confidence Interval (CI), 52% to 96%], overall. After 3 years, efficacy was estimated to be 67% (90% CI, 40% to 82%) among children who were 4-16 years of age at the time of vaccination and 8% (90% CI, -102% to 58%) among children who were 1-3 years of age at the time of vaccination. (5)
The efficacy of a serogroup C meningococcal vaccine in infants and young children was evaluated in a placebo-controlled trial during a serogroup C epidemic in Brazil. Vaccine efficacy was estimated to be 12% (95% CI, -55% to 62%) among children 6 to 23 months of age and 55% (95% CI, -4% to 72%) among children 24 to 36 months of age. (6)
8.5. Geriatric Use
Clinical studies of Menomune – A/C/Y/W-135 vaccine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
11. DESCRIPTION
Menomune – A/C/Y/W-135, Meningococcal Polysaccharide Vaccine, Groups A, C, Y and W-135 Combined, is a vaccine for subcutaneous injection. Menomune – A/C/Y/W-135 vaccine consists of a sterile lyophilized preparation of the group-specific polysaccharide antigens from N meningitidis, Group A, Group C, Group Y, and Group W-135. N meningitidis are cultivated on Mueller Hinton casein agar (7) and grown in Watson Scherp casamino acid media (8). The purified polysaccharide is extracted from the N meningitidis cells and separated from the media by procedures which include centrifugation, detergent precipitation, alcohol precipitation, solvent or organic extraction, and diafiltration. No preservative is added during manufacture.
The diluent (0.6 mL) for the single dose presentation contains sterile, pyrogen-free distilled water without preservative. The diluent (6 mL) for the multidose presentation contains sterile, pyrogen-free distilled water and thimerosal, a mercury derivative, which is added as a preservative for the reconstituted vaccine. [See How Supplied/Storage and Handling (16) .]
After reconstitution with diluent, the vaccine is a clear colorless liquid solution. Each 0.5 mL dose contains 50 mcg of polysaccharide from each of serogroups A, C, Y, and W-135. Reconstituted vaccine from a multidose vial also contains 25 mcg mercury per dose.
Each dose of vaccine contains 2.5 mg to 5 mg of lactose added as a stabilizer. (9)
Potency is evaluated by measuring the molecular size of each polysaccharide component using a column chromatography method as standardized by the US Food and Drug Administration (FDA) and the World Health Organization (WHO) (10) for Meningococcal Polysaccharide Vaccine.
12. CLINICAL PHARMACOLOGY
12.1. Mechanism of Action
The presence of bactericidal anti-capsular meningococcal antibodies has been associated with protection from invasive meningococcal disease. (11) (12) Menomune – A/C/Y/W-135 vaccine induces the production of bactericidal antibodies specific to the capsular polysaccharides of serogroups A, C, Y, and W-135.
13. NON-CLINICAL TOXICOLOGY
13.1. Carcinogenesis, mutagenesis, impairment of fertility
Menomune – A/C/Y/W-135 vaccine has not been evaluated for carcinogenic or mutagenic potential or impairment of fertility.
14. CLINICAL STUDIES
Effectiveness of Menomune – A/C/Y/W-135 vaccine was inferred by evaluating the proportion of children 2-10 years of age achieving a pre-specified level of serum bactericidal antibody and the proportion of persons 11-55 years of age achieving a 4-fold increase from baseline in serum bactericidal antibody, for each serogroup. Evidence for clinical efficacy against serogroup-specific meningococcal disease in school-age children and adults has been obtained from historical field trials and observational studies with other high molecular weight polysaccharide vaccines containing meningococcal serogroup A and/or C components. (6) No studies have been conducted to evaluate the efficacy of meningococcal polysaccharide vaccines against disease due to serogroups Y and W-135.
Menomune – A/C/Y/W-135 vaccine was used as the control vaccine in three US, multi-center, clinical trials designed primarily to evaluate the immunogenicity and safety of Menactra vaccine in children (2-10 years old), adolescents (11-18 years old), and adults (18-55 years old), respectively. In these trials, participants in the control arm received a dose of Menomune – A/C/Y/W-135 vaccine. Sera were obtained before and approximately 28 days after vaccination. The Serum Bactericidal Assay (SBA) used to test sera contained an exogenous complement source that was either human (SBA-H) or, when correlated to SBA-H, baby rabbit (SBA-BR). (13)
Data on immune responses, as measured by SBA-H, following Menomune – A/C/Y/W-135 vaccine in a subset of children 2-10 years old are presented in Table 3. Data on immune responses, as measured by SBA-BR, following Menomune – A/C/Y/W-135 vaccine in adolescents and adults are presented in Table 4.
| Menomune – A/C/Y/W-135 vaccine Aged 2-3 Years N  |
Menomune – A/C/Y/W-135 vaccine Aged 4-10 Years N  |
||||||
|---|---|---|---|---|---|---|---|
| Serogroup | (95% CI)  |
Serogroup | (95% CI)  |
||||
| The study was designed to show the safety and immunogenicity of Menactra vaccine are non-inferior to that of Menomune – A/C/Y/W-135 vaccine. The table shows the immune response in Menomune – A/C/Y/W-135 vaccine participants from this study. | |||||||
| A | % ≥1:8 | 64 | 50,77 | A | % ≥1:8 | 55 | 44,66 |
| GMT | 10 | 7,12 | GMT | 7 | 6,9 | ||
| C | % ≥1:8 | 38 | 25,53 | C | % ≥1:8 | 48 | 37,59 |
| GMT | 11 | 5,21 | GMT | 12 | 7,18 | ||
| Y | % ≥1:8 | 73 | 59,84 | Y | % ≥1:8 | 92 | 84,97 |
| GMT | 18 | 11,27 | GMT | 46 | 33,66 | ||
| W-135 | % ≥1:8 | 33 | 20,47 | W-135 | % ≥1:8 | 79 | 68,87 |
| GMT | 5 | 3,6 | GMT | 20 | 14,27 | ||
In participants 2-3 years of age with undetectable pre-vaccination SBA titers (ie, <4 at Day 0), rates of seroconversion (defined as SBA titer ≥8 at Day 28) following Menomune – A/C/Y/W-135 vaccine were 55%, serogroup A (n=16/29); 30%, serogroup C (n=13/43); 57%, serogroup Y (n=17/30); 26%, serogroup W-135 (n=11/43).
In participants 4-10 years of age with undetectable pre-vaccination SBA titers (ie, <4 at Day 0), rates of seroconversion (defined as SBA titer ≥8 at Day 28) following Menomune – A/C/Y/W-135 vaccine were 48%, serogroup A (n=10/21); 38%, serogroup C (n=19/50); 84%, serogroup Y (n=38/45); 68%, serogroup W-135 (n=26/38).
| Menomune – A/C/Y/W-135 vaccine Aged 11-18 Years N  |
Menomune – A/C/Y/W-135 vaccine Aged 18-55 Years N  |
||||||
|---|---|---|---|---|---|---|---|
| Serogroup | (95% CI)  |
Serogroup | (95% CI)  |
||||
| Both studies (11-18 and 18-55 years of age) were designed to show the safety and immunogenicity of Menactra vaccine are non-inferior to that of Menomune – A/C/Y/W-135 vaccine. The table shows the immune response in Menomune – A/C/Y/W-135 vaccine participants from these studies. | |||||||
| A | % ≥4-fold rise |
92.4 | (89.5, 94.8) | A | % ≥4-fold rise |
84.6 | (82.3, 86.7) |
| GMT | 3246 | (2910, 3620) | GMT | 4114 | (3832, 4417) | ||
| C | % ≥4-fold rise |
88.7 | (85.2, 91.5) | C | % ≥4-fold rise |
89.7 | (87.8, 91.4) |
| GMT | 1639 | (1406, 1911) | GMT | 3469 | (3148, 3823) | ||
| Y | % ≥4-fold rise |
80.1 | (76.0, 83.8) | Y | % ≥4-fold rise |
79.4 | (76.9, 81.8) |
| GMT | 1228 | (1088, 1386) | GMT | 2449 | (2237, 2680) | ||
| W-135 | % ≥4-fold rise |
95.3 | (92.8, 97.1) | W-135 | % ≥4-fold rise |
94.4 | (92.8, 95.6) |
| GMT | 1545 | (1384, 1725) | GMT | 1871 | (1723, 2032) | ||
In participants 11-18 years of age with undetectable pre-vaccination SBA titers (ie, <8 at Day 0), rates of seroconversion (defined as a ≥4-fold rise in Day 28 SBA titers) following Menomune – A/C/Y/W-135 vaccine were 100%, serogroup A (n=93/93); 99%, serogroup C (n=151/152); 100%, serogroup Y (n=47/47); 99%, serogroup W-135 (n=138/139).
In participants 18-55 years of age with undetectable pre-vaccination SBA titers (ie, <8 at Day 0), rates of seroconversion (defined as a ≥4-fold rise in Day 28 SBA titers) following Menomune – A/C/Y/W-135 vaccine were 99%, serogroup A (n=143/144); 98%, serogroup C (n=297/304); 97%, serogroup Y (n=221/228); 99%, serogroup W-135 (n=325/328).
15. REFERENCES
-
1 Centers for Disease Control and Prevention. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2005;54 (No. RR - 7): 1-21. -
2 Centers for Disease Control and Prevention. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR 2007;56:794-5. -
3 Centers for Disease Control and Prevention. Recommendation from the Advisory Committee on Immunization Practices (ACIP) for use of quadrivalent meningococcal conjugate vaccine (MCV4) in children aged 2-10 years at increased risk for invasive meningococcal disease. MMWR 2007;56:1265-6. -
4 Recommendations of the Advisory Committee on Immunization Practices (ACIP) General Recommendations on Immunization. MMWR 2006 December 01;55(RR15):1-48. -
5 Reingold AL, et al. Age-specific differences in duration of clinical protection after vaccination with meningococcal polysaccharide A vaccine. Lancet. 1985;No.8447:114-118. -
6 Granoff DM, et al. Meningococcal vaccines. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5th ed. Philadelphia, PA: WB Saunders Company; 2008:399-434. -
7 Mueller H, et al. A protein-free medium for primary isolation of the gonococcus and meningococcus. Proc Soc Exp Biol Med 1941;48:330. -
8 Watson RG, et al. The specific hapten of group C (group II a) meningococcus. II. Chemical nature. J Immunol. 1958;81:337. -
9 Tiesjema RH, et al. Enhanced stability of meningococcal polysaccharide vaccines by using lactose as a menstruum for lyophilization. Bull WHO 1977;55:43-48. -
10 WHO Technical Report Series, 1981;No.658. -
11 Mäkelä PH, et al. Evolution of conjugate vaccines. Expert Rev Vaccines 2002;1(3):399-410. -
12 Goldschneider I, et al. Human immunity to the meningococcus. I. The Role of Humoral Antibodies. J Exp Med 1969;129:1307-1326. -
13 Maslanka SE, et al. Standardization and a Multilaboratory Comparison of Neisseria meningitidis Serogroup A and C Serum Bactericidal Assays. Clin and Diag Lab Immunol 1997;156-167.
16. HOW SUPPLIED/STORAGE AND HANDLING
16.1. How Supplied
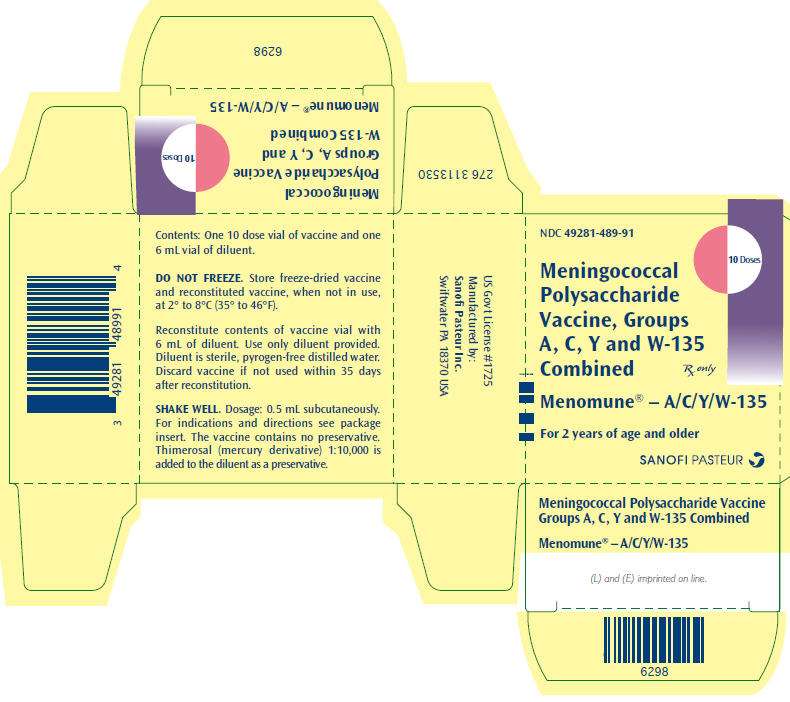
One single dose vial of lyophilized vaccine (NDC 49281-487-58) with one 0.6 mL vial of diluent (NDC 49281-466-08) (contains no preservative). Supplied as package NDC 49281-489-01.
One 10 dose vial of lyophilized vaccine (NDC 49281-488-78) with one 6.0 mL vial of diluent (NDC 49281-466-91) (contains preservative). Supplied as package NDC 49281-489-91.
16.2. Storage
Store lyophilized vaccine, diluent, and reconstituted vaccine, when not in use, at 2° to 8°C (35° to 46°F). Do not freeze.
Do not use after the expiration date shown on the vial labels of the lyophilized vaccine and diluent.
Discard remainder of reconstituted vaccine from multidose vials within 35 days after reconstitution. Vaccine from single dose vials should be used immediately after reconstitution.
17. PATIENT COUNSELING INFORMATION
Before administration of Menomune – A/C/Y/W-135 vaccine, health-care providers should inform the patient, parent or guardian of the benefits and risks of the vaccine. The health-care provider should provide the Vaccine Information Statements, which are required by the National Childhood Vaccine Injury Act of 1986 to be given with each immunization. Patients, parents, or guardians should be instructed to report adverse reactions to their health-care provider.
Rx only
Menomune® – A/C/Y/W-135 is a registered trademark of sanofi pasteur and its subsidiaries.
Manufactured by:
Sanofi Pasteur Inc.
Swiftwater PA 18370 USA
6344
PRINCIPAL DISPLAY PANEL - Multidose Vial Label
NDC 49281-488-78
10
Doses
Meningococcal
Polysaccharide
Vaccine, Groups
A, C, Y and W-135
Combined
Rx only
Menomune® – A/C/Y/W-135

PRINCIPAL DISPLAY PANEL - Diluent Vial Label
NDC 49281-466-91
6 mL
Diluent for
Reconstitution
of Meningococcal
Polysaccharide
Vaccine
Rx only

PRINCIPAL DISPLAY PANEL - Multidose Kit Carton
NDC 49281-489-91
10 Doses
Meningococcal
Polysaccharide
Vaccine, Groups
A, C, Y and W-135
Combined
Rx only
Menomune® – A/C/Y/W-135
For 2 years of age and older
SANOFI PASTEUR
MENOMUNE - A/C/Y/W-135 COMBINEDNeisseria Meningitidis Group A Capsular Polysaccharide Antigen, Neisseria Meningitidis Group C Capsular Polysaccharide Antigen, Neisseria Meningitidis Group Y Capsular Polysaccharide Antigen, and Neisseria Meningitidis Group W-135 Capsular Polysaccharide Antigen KIT
| ||||||||||||||||||||||||||||||||||||||||