Lovastatin
FULL PRESCRIBING INFORMATION: CONTENTS*
- LOVASTATIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- CLINICAL STUDIES IN ADULTS
- INDICATIONS & USAGE
- LOVASTATIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- LOVASTATIN ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
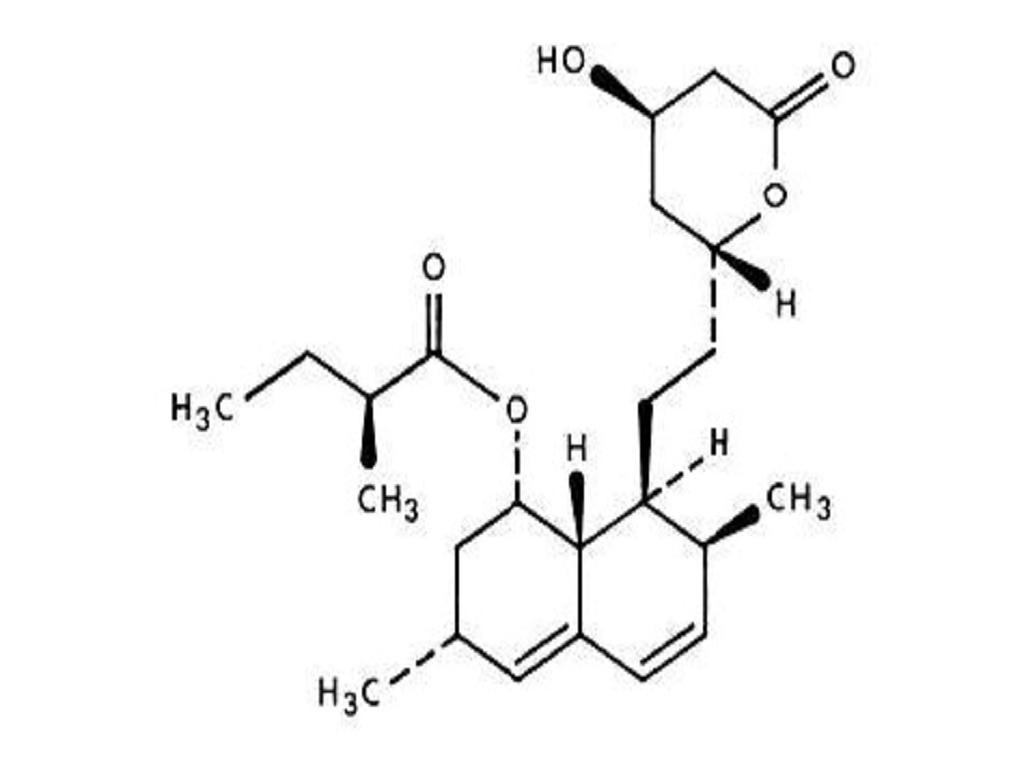
LOVASTATIN DESCRIPTION
CLINICAL PHARMACOLOGY
Tables IIIIClinical Studies
PHARMACOKINETICS
PRECAUTIONSGeriatric Use
WARNINGSMyopathy/RhabdomyolysisPRECAUTIONSDrug Interactions
PRECAUTIONSDrug Interactions
CLINICAL STUDIES IN ADULTS
Tables IIII
Table I
Table II
Expanded Clinical Evaluation of Lovastatin (EXCEL) Study
Table III
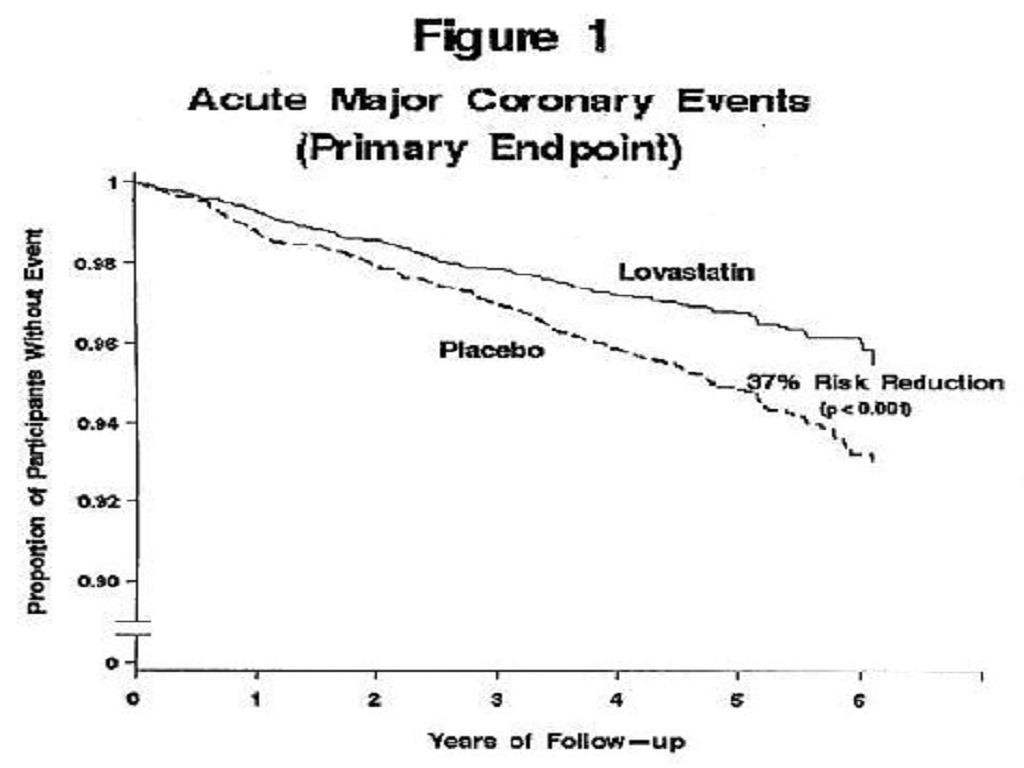
Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS)
Figure 1risk factors had risk reductions (RR) in both acute major coronary events (RR 43%) and coronary revascularization procedures (RR 37%). Because there were too few events among those participants with age as their only risk factor in this study, the effect of lovastatin on outcomes could not be adequately assessed in this subgroup.
Atherosclerosis
In the Canadian Coronary Atherosclerosis Intervention Trial (CCAIT), the effect of therapy with lovastatin on coronary atherosclerosis was assessed by coronary angiography in hyperlipidemic patients. In the randomized, double-blind, controlled clinical trial, patients were treated with conventional measures (usually diet and 325 mg of aspirin every other day) and either lovastatin 20-80 mg daily or placebo. Angiograms were evaluated at baseline and at two years by computerized quantitative coronary angiography (QCA). Lovastatin significantly slowed the progression of lesions as measured by the mean change per-patient in minimum lumen diameter (the primary endpoint) and percent diameter stenosis, and decreased the proportions of patients categorized with disease progression (33% vs. 50%) and with new lesions (16% vs. 32%).
In a similarly designed trial, the Monitored Atherosclerosis Regression Study (MARS), patients were treated with diet and either lovastatin 80 mg daily or placebo. No statistically significant difference between lovastatin and placebo was seen for the primary endpoint (mean change per patient in percent diameter stenosis of all lesions), or for most secondary QCA endpoints. Visual assessment by angiographers who formed a consensus opinion of overall angiographic change (Global Change Score) was also a secondary endpoint. By this endpoint, significant slowing of disease was seen, with regression in 23% of patients treated with lovastatin compared to 11% of placebo patients.
In the Familial Atherosclerosis Treatment Study (FATS), either lovastatin or niacin in combination with a bile acid sequestrant for 2.5 years in hyperlipidemic subjects significantly reduced the frequency of progression and increased the frequency of regression of coronary atherosclerotic lesions by QCA compared to diet and, in some cases, low-dose resin.
The effect of lovastatin on the progression of atherosclerosis in the coronary arteries has been corroborated by similar findings in another vasculature. In the Asymptomatic Carotid Artery Progression Study (ACAPS), the effect of therapy with lovastatin on carotid atherosclerosis was assessed by B-mode ultrasonography in hyperlipidemic patients with early carotid lesions and without known coronary heart disease at baseline. In this double-blind, controlled clinical trial, 919 patients were randomized in a 2 x 2 factorial design to placebo, lovastatin 10-40 mg daily and/or warfarin. Ultrasonograms of the carotid walls were used to determine the change per patient from baseline to three years in mean maximum intimal-medial thickness (IMT) of 12 measured segments. There was a significant regression of carotid lesions in patients receiving lovastatin alone compared to those receiving placebo alone (p=0.001). The predictive value of changes in IMT for stroke has not yet been established. In the lovastatin group there was a significant reduction in the number of patients with major cardiovascular events relative to the placebo group (5 vs. 14) and a significant reduction in all-cause mortality (1 vs. 8).
Eye
There was a high prevalence of baseline lenticular opacities in the patient population included in the early clinical trials with lovastatin. During these trials the appearance of new opacities was noted in both the lovastatin and placebo groups. There was no clinically significant change in visual acuity in the patients who had new opacities reported nor was any patient, including those with opacities noted at baseline, discontinued from therapy because of a decrease in visual acuity.
A three-year, double-blind, placebo-controlled study in hypercholesterolemic patients to assess the effect of lovastatin on the human lens demonstrated that there were no clinically or statistically significant differences between the lovastatin and placebo groups in the incidence, type or progression of lenticular opacities. There are no controlled clinical data assessing the lens available for treatment beyond three years.
Clinical Studies in Adolescent Patients
Efficacy of Lovastatin in Adolescent Boys with Heterozygous Familial Hypercholesterolemia
In a double-blind, placebo-controlled study, 132 boys 10-17 years of age (mean age 12.7 yrs) with heterozygous familial hypercholesterolemia (heFH) were randomized to lovastatin (n=67) or placebo (n=65) for 48 weeks. Inclusion in the study required a baseline LDL-C level between 189 and 500 mg/dL and at least one parent with an LDL-C level >189 mg/dL. The mean baseline LDL-C value was 253.1 mg/dL (range: 171-379 mg/dL) in the lovastatin group compared to 248.2 mg/dL (range 158.5-413.5 mg/dL) in the placebo group. The dosage of lovastatin (once daily in the evening) was 10 mg for the first 8 weeks, 20 mg for the second 8 weeks, and 40 mg thereafter.
Lovastatin significantly decreased plasma levels of total-C, LDL-C and apolipoprotein B (seeTable IV).
TABLE IV Lipid-lowering Effects of Lovastatin in Adolescent Boys with Heterozygous Familial Hypercholesterolemia (Mean Percent Change from Baseline at week 48 in Intention-to-Treat Population)
*data presented as median percent changesDOSAGENTOTAL-CLDL-CHDL-CTG. *Apolipoprotein BPlacebo61-1.1-1.4-2.2-1.4-4.4Lovastatin64-19.3-24.2+1.1-1.9-21The mean achieved LDL-C value was 190.9 mg/dL (range: 108-336 mg/dL) in the lovastatin group compared to 244.8 mg/dL (range: 135-404 mg/dL) in the placebo group.
Efficacy of Lovastatin in Post-menarchal Girls with Heterozygous Familial Hypercholesterolemia
In a double-blind, placebo-controlled study, 54 girls 10-17 years of age who were at least 1 year post-menarche with heFH were randomized to lovastatin (n=35) or placebo (n=19) for 24 weeks. Inclusion in the study required a baseline LDL-C level of 160-400 mg/dL and a parental history of familial hypercholesterolemia. The mean baseline LDL-C value was 218.3 mg/dL (range: 136.3-363.7 mg/dL) in the lovastatin group compared to 198.8 mg/dL (range: 151.5-283.1 mg/dL) in the placebo group. The dosage of lovastatin (once daily in the evening) was 20 mg for the first 4 weeks, and 40 mg thereafter.
Lovastatin significantly decreased plasma levels of total-C, LDL-C, and apolipoprotein B (seeTable V).
TABLE V Lipid-lowering Effects of Lovastatin in Post-menarchal Girls with Heterozygous Familial Hypercholesterolemia (Mean Percent Change from Baseline at Week 24 in Intention-to-Treat Population)
*data presented as median percent changesDOSAGENTOTAL-CLDL-CHDL-CTG. *Apolipoprotein BPlacebo18+3.6+2.5+4.8-3.0+6.4Lovastatin35-22.4-29.2+2.4-22.7-24.4The mean achieved LDL-C value was 154.5 mg/dL (range: 82-286 mg/dL) in the lovastatin group compared to 203.5 mg/dL (range: 135-304 mg/dL) in the placebo group.
The safety and efficacy of doses above 40 mg daily have not been studied in children. The long-term efficacy of lovastatin therapy in childhood to reduce morbidity and mortality in adulthood has not been established.
INDICATIONS & USAGE
-
● Myocardial infarction
-
● Unstable angina
-
● Coronary revascularization procedures
Hypercholesterolemia
Adolescent Patients with Heterozygous Familial Hypercholesterolemia
-
● there is a positive family history of premature cardiovascular disease or
-
● two or more other CVD risk factors are present in the adolescent patient
General Recommendations
LOVASTATIN CONTRAINDICATIONS
WARNINGS
Lovastatin should be administered to women of childbearing age only when such patients are highly unlikely to conceive.PRECAUTIONSPregnancy
WARNINGS
Myopathy/RhabdomyolysisAs with other HMG-CoA reductase inhibitors, the risk of myopathy/rhabdomyolysis is dose related
All patients starting therapy with lovastatin, or whose dose of lovastatin is being increased, should be advised of the risk of myopathy and told to report promptly any unexplained muscle pain, tenderness or weakness. Lovastatin therapy should be discontinued immediately if myopathy is diagnosed or suspected.
The risk of myopathy/rhabdomyolysis is increased by concomitant use of lovastatin with the following:
Potent inhibitors of CYP3A4:
The use of lovastatin concomitantly with the potent CYP3A4 inhibitors itraconazole, ketoconazole, erythromycin, clarithromycin, telithromycin, HIV protease inhibitors, nefazodone, or large quantities of grapefruit juice (>1 quart daily) should be avoided.
Gemfibrozil, particularly with higher doses of lovastatin: The dose of lovastatin should not exceed 20 mg daily in patients receiving concomitant medication with gemfibrozil. The combined use of lovastatin with gemfibrozil should be avoided, unless the benefits are likely to outweigh the increased risks of this drug combination.
Other lipid-lowering drugs (other fibrates org/day of niacin): The dose of lovastatin should not exceed 20 mg daily in patients receiving concomitant medication with other fibrates org/day of niacin.
Cyclosporine or danazol, with higher doses of lovastatin: The dose of lovastatin should not exceed 20 mg daily in patients receiving concomitant medication with cyclosporine or danazol.
Amiodarone or verapamil: The dose of lovastatin should not exceed 40 mg daily in patients receiving concomitant medication with amiodarone or verapamil.
Table VIalso CLINICAL PHARMACOLOGY, PharmacokineticsPRECAUTIONS, Drug InteractionsDOSAGE AND ADMINISTRATION).
Table VI Drug Interactions Associated with Increased Risk of Myopathy/Rhabdomyolysis
Interacting AgentsPrescribing RecommendationsItraconazole Ketoconazole Erythromycin Clarithromycin Telithromycin HIV protease inhibitors NefazodoneAvoid lovastatinGemfibrozil Other fibrates Lipid-lowering doses (g/day) of niacin Cyclosporine DanazolDo not exceed 20 mg lovastatin dailyAmiodarone VerapamilDo not exceed 40 mg lovastatin dailyGrapefruit juiceAvoid large quantities of grapefruit juice (quart daily)
Liver Dysfunction
Persistent increases (to more than 3 times the upper limit of normal) in serum transaminases occurred in 1.9% of adult patients who received lovastatin for at least one year in early clinical trials(seeADVERSE REACTIONS). When the drug was interrupted or discontinued in these patients, the transaminase levels usually fell slowly to pretreatment levels. The increases usually appeared 3 to 12 months after the start of therapy with lovastatin, and were not associated with jaundice or other clinical signs or symptoms. There was no evidence of hypersensitivity. In the EXCEL study (seeCLINICAL PHARMACOLOGY,Clinical Studies), the incidence of persistent increases in serum transaminases over 48 weeks was 0.1% for placebo, 0.1% at 20 mg/day, 0.9% at 40 mg/day, and 1.5% at 80 mg/day in patients on lovastatin. However, in post-marketing experience with lovastatin, symptomatic liver disease has been reported rarely at all dosages (seeADVERSE REACTIONS).
In AFCAPS/TexCAPS, the number of participants with consecutive elevations of either alanine aminotransferase (ALT) or aspartate aminotransferase (AST) (> 3 times the upper limit of normal), over a median of 5.1 years of follow-up, was not significantly different between the lovastatin and placebo groups (18 [0.6%] vs. 11 [0.3%]). The starting dose of lovastatin was 20 mg/day; 50% of the lovastatin treated participants were titrated to 40 mg/day at Week 18. Of the 18 participants on lovastatin with consecutive elevations of either ALT or AST, 11 (0.7%) elevations occurred in participants taking 20 mg/day, while 7 (0.4%) elevations occurred in participants titrated to 40 mg/day. Elevated transaminases resulted in discontinuation of 6 (0.2%) participants from therapy in the lovastatin group (n=3,304) and 4 (0.1%) in the placebo group (n=3,301).
It is recommended that liver function tests be performed prior to initiation of therapy in patients with a history of liver disease, or when otherwise clinically indicated. It is recommend that liver function tests be performed in all patients prior to use of 40mg or more daily and thereafter when clinically indicated. Patients who develop increased transaminase levels should be monitored with a second liver function evaluation to confirm the finding and be followed thereafter with frequent liver function tests until the abnormality (ies) return to normal. Should an increase in AST or ALT of three times the upper limit of normal or greater persist, withdrawal of therapy with lovastatin is recommended.
The drug should be used with caution in patients who consume substantial quantities of alcohol and/or have a past history of liver disease. Active liver disease or unexplained transaminase elevations are contraindications to the use of lovastatin.
As with other lipid-lowering agents, moderate (less than three times the upper limit of normal) elevations of serum transaminases have been reported following therapy with lovastatin (seeADVERSE REACTIONS). These changes appeared soon after initiation of therapy with lovastatin, were often transient, were not accompanied by any symptoms and interruption of treatment was not required.
PRECAUTIONS
GeneralWARNINGSADVERSE REACTIONS
Homozygous Familial Hypercholesterolemia
ADVERSE REACTIONS
INFORMATION FOR PATIENTS
Patients should be advised about substances they should not take concomitantly with lovastatin and be advised to report promptly unexplained muscle pain, tenderness, or weakness (see list below andWARNINGS,Myopathy/Rhabdomyolysis). Patients should also be advised to inform other physicians prescribing a new medication that they are taking lovastatin.DRUG INTERACTIONS
CYP3A4 InteractionsWARNINGSMyopathy/RhabdomyolysisCLINICAL PHARMACOLOGYPharmacokinetics
Itraconazole
Ketoconazole
Erythromycin
Clarithromycin
Telithromycin
HIV protease inhibitors
Nefazodone
Cyclosporine
Large quantities of grapefruit juice (>1 quart daily)
Interactions with lipid-lowering drugs that can cause myopathy when given alone
SeeWARNINGS,Myopathy/Rhabdomyolysis.
Gemfibrozil
Other fibrates
Niacin (nicotinic acid) (g/day)
Other drug interactions
WARNINGMyopathy/Rhabdomyolysis
WARNINGSMyopathy/RhabdomyolysisCLINICAL PHARMACOLOGY,Pharmacokinetics).
Coumarin Anticoagulants: In a small clinical trial in which lovastatin was administered to warfarin treated patients; no effect on prothrombin time was detected. However, another HMG-CoA reductase inhibitor has been found to produce a less than two seconds increase in prothrombin time in healthy volunteers receiving low doses of warfarin. Also, bleeding and/or increased prothrombin time have been reported in a few patients taking coumarin anticoagulants concomitantly with lovastatin. It is recommended that in patients taking anticoagulants, prothrombin time be determined before starting lovastatin and frequently enough during early therapy to insure that no significant alteration of prothrombin time occurs. Once a stable prothrombin time has been documented, prothrombin times can be monitored at the intervals usually recommended for patients on coumarin anticoagulants. If the dose of lovastatin is changed, the same procedure should be repeated. Lovastatin therapy has not been associated with bleeding or with changes in prothrombin time in patients not taking anticoagulants.
Propranolol: In normal volunteers, there was no clinically significant pharmacokinetic or pharmacodynamic interaction with concomitant administration of single doses of lovastatin and propranolol.
Digoxin: In patients with hypercholesterolemia, concomitant administration of lovastatin and digoxin resulted in no effect on digoxin plasma concentrations.
Oral Hypoglycemic Agents: In pharmacokinetic studies of lovastatin in hypercholesterolemic non-insulin dependent diabetic patients, there was no drug interaction with glipizide or with chlorpropamide (seeCLINICAL PHARMACOLOGY,Clinical Studies).
Endocrine Function
HMG-CoA reductase inhibitors interfere with cholesterol synthesis and as such might theoretically blunt adrenal and/or gonadal steroid production. Results of clinical trials with drugs in this class have been inconsistent with regard to drug effects on basal and reserve steroid levels. However, clinical studies have shown that lovastatin does not reduce basal plasma cortisol concentration or impair adrenal reserve, and does not reduce basal plasma testosterone concentration. Another HMG-CoA reductase inhibitor has been shown to reduce the plasma testosterone response to HCG. In the same study, the mean testosterone response to HCG was slightly but not significantly reduced after treatment with lovastatin 40 mg daily for 16 weeks in 21 men. The effects of HMG-CoA reductase inhibitors on male fertility have not been studied in adequate numbers of male patients. The effects, if any, on the pituitary-gonadal axis in premenopausal women are unknown. Patients treated with lovastatin who develop clinical evidence of endocrine dysfunction should be evaluated appropriately. Caution should also be exercised if an HMG-CoA reductase inhibitor or other agent used to lower cholesterol levels is administered to patients also receiving other drugs (e.g., ketoconazole, spironolactone, cimetidine) that may decrease the levels or activity of endogenous steroid hormones.
CNS Toxicity
Lovastatin produced optic nerve degeneration (Wallerian degeneration of retinogeniculate fibers) in clinically normal dogs in a dose-dependent fashion starting at 60 mg/kg/day, a dose that produced mean plasma drug levels about 30 times higher than the mean drug level in humans taking the highest recommended dose (as measured by total enzyme inhibitory activity). Vestibulocochlear Wallerian-like degeneration and retinal ganglion cell chromatolysis were also seen in dogs treated for 14 weeks at 180 mg/kg/day, a dose which resulted in a mean plasma drug level (C max) similar to that seen with the 60 mg/kg/day dose.
CNS vascular lesions, characterized by perivascular hemorrhage and edema, mononuclear cell infiltration of perivascular spaces, perivascular fibrin deposits and necrosis of small vessels, were seen in dogs treated with lovastatin at a dose of 180 mg/kg/day, a dose which produced plasma drug levels (C max) which were about 30 times higher than the mean values in humans taking 80 mg/day.
Similar optic nerve and CNS vascular lesions have been observed with other drugs of this class.
Cataracts were seen in dogs treated for 11 and 28 weeks at 180 mg/kg/day and 1 year at 60 mg/kg/day.
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Pregnancy Category XCONTRAINDICATIONS
CONTRAINDICATIONS
NURSING MOTHERS
CONTRAINDICATIONSPEDIATRIC USE
Doses greater than 40 mg have not been studied in this population.CLINICAL PHARMACOLOGYClinical Studies in Adolescent PatientsADVERSE REACTIONS,Adolescent PatientsDOSAGE AND ADMINISTRATION,Adolescent Patients (10-17 years of age) with Heterozygous Familial Hypercholesterolemia. Adolescent females should be counseled on appropriate contraceptive methods while on lovastatin therapy (seeCONTRAINDICATIONSandPRECAUTIONS,Pregnancy).Lovastatin has not been studied in pre-pubertal patients or patients younger than 10 years of age.GERIATRIC USE
CLINICAL PHARMACOLOGYLOVASTATIN ADVERSE REACTIONS
Phase III Clinical Studies
Expanded Clinical Evaluation of Lovastatin [EXCEL] Study
WARNINGSLiver DysfunctionWARNINGSMyopathy/Rhabdomyolysis
Expanded Clinical Evaluation of Lovastatin (EXCEL) Study
CLINICAL PHARMACOLOGYClinical Studies
Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS)
CLINICAL PHARMACOLOGYClinical StudiesADVERSE REACTIONSExpanded Clinical Evaluation of Lovastatin (EXCEL) Study
Concomitant Therapy
WARNINGSMyopathy/Rhabdomyolysis
Adolescent Patients (ages 10-17 years)
CLINICAL PHARMACOLOGYClinical Studies in Adolescent PatientsPRECAUTIONSPediatric Use
OVERDOSAGE
DOSAGE & ADMINISTRATION
Adult Patients
CLINICAL PHARMACOLOGYINDICATIONS AND USAGE
Dosage in Patients taking Cyclosporine or Danazol
WARNINGSMyopathy/Rhabdomyolysis
Dosage in Patients taking Amiodarone or Verapamil
WARNINGSMyopathy/RhabdomyolysisPRECAUTIONSDrug InteractionsOther drug interactions
Adolescent Patients (10-17 years of age) with Heterozygous Familial Hypercholesterolemia
CLINICAL PHARMACOLOGYINDICATIONS AND USAGE
Concomitant Lipid-Lowering Therapy
WARNINGSMyopathy/RhabdomyolysisPRECAUTIONSDrug Interactions
Dosage in Patients with Renal Insufficiency
CLINICAL PHARMACOLOGYWARNINGSMyopathy/Rhabdomyolysis
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

LovastatinLovastatin TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!