Lidocaine Hydrochloride and Dextrose
Lidocaine Hydrochloride and 5% Dextrose Injection, USP in Plastic Container VIAFLEX Plus Container
FULL PRESCRIBING INFORMATION: CONTENTS*
- LIDOCAINE HYDROCHLORIDE AND DEXTROSE DESCRIPTION
- CLINICAL PHARMACOLOGY
- LIDOCAINE HYDROCHLORIDE AND DEXTROSE INDICATIONS AND USAGE
- LIDOCAINE HYDROCHLORIDE AND DEXTROSE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- LIDOCAINE HYDROCHLORIDE AND DEXTROSE ADVERSE REACTIONS
- OVERDOSAGE
- LIDOCAINE HYDROCHLORIDE AND DEXTROSE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- DIRECTIONS FOR USE OF VIAFLEX PLUS PLASTIC CONTAINER
FULL PRESCRIBING INFORMATION
LIDOCAINE HYDROCHLORIDE AND DEXTROSE DESCRIPTION
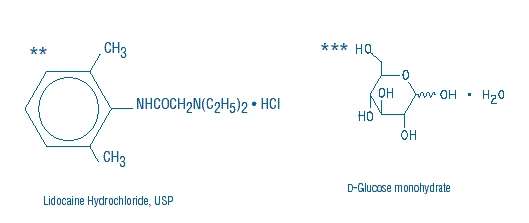
Lidocaine Hydrochloride and 5% Dextrose Injection, USP is a sterile, nonpyrogenic solution prepared from lidocaine hydrochloride and dextrose in water for injection. It contains no antimicrobial agents. Lidocaine hydrochloride is designated chemically as 2-(Diethylamino) - 2', 6' - acetoxylidide monohydrochloride. The solution serves as a cardiac antiarrhythmic agent intended for intravenous use. Composition, osmolarity, pH and caloric content are shown in Table 1. The pH is adjusted with sodium hydroxide.
| Composition |
 |
pH | Caloric Content (kcal/L) | ||
| **Lidocaine Hydrochloride, USP (mg/mL) |
***Dextrose Hydrous, USP (g/L) |
||||
| 0.4% Lidocaine Hydrochloride and 5% Dextrose Injection, USP | 4 | 50 | 282 | 4.0 (3.0 to 7.0) |
170 |
| 0.8% Lidocaine Hydrochloride and 5% Dextrose Injection, USP | 8 | 50 | 311 | 4.0 (3.0 to 7.0) |
170 |
This VIAFLEX Plus plastic container is fabricated from a specially formulated polyvinyl chloride (PL 146 Plastic). VIAFLEX Plus on the container indicates the presence of a drug additive in a drug vehicle. The VIAFLEX Plus plastic container system utilizes the same container as the VIAFLEX plastic container system. The amount of water that can permeate from inside the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the plastic container can leach out certain of its chemical components in very small amounts within the expiration period, e.g., di-2-ethylhexyl phthalate (DEHP), up to 5 parts per million. However, the safety of the plastic has been confirmed in tests in animals according to USP biological standards for plastic containers as well as by tissue culture toxicity studies.
CLINICAL PHARMACOLOGY
Lidocaine hydrochloride exerts an antiarrhythmic effect by increasing the electrical stimulation threshold of the ventricle during diastole. In usual therapeutic doses, lidocaine hydrochloride produces no change in myocardial contractility, in systemic arterial pressure, or in absolute refractory period.
About 90% of an administered dose of the drug is metabolized in the liver. The remaining 10% is excreted unchanged via the kidneys.
Lidocaine toxicity is related to systemic blood levels. The decreased clearance and longer half-life of lidocaine should be taken into consideration with prolonged (24 hour) infusions. Constant rate of infusion may result in toxic accumulation of lidocaine. Infusion should be reduced approximately one-half to compensate for decreased rate of clearance and concomitant or prior administration of propranolol may further increase blood concentrations by as much as 30% (See Drug Interactions).
LIDOCAINE HYDROCHLORIDE AND DEXTROSE INDICATIONS AND USAGE
Lidocaine hydrochloride administered intravenously is specifically indicated in the acute management of (1) ventricular arrhythmias occurring during cardiac manipulations, such as cardiac surgery and (2) life-threatening arrhythmias which are ventricular in origin, such as occur during acute myocardial infarction.
LIDOCAINE HYDROCHLORIDE AND DEXTROSE CONTRAINDICATIONS
Lidocaine hydrochloride is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type. Lidocaine should not be used in patients with Stokes-Adams syndrome, Wolff-Parkinson-White syndrome, or with severe degrees of sinoatrial, atrioventricular, or intraventricular block.
Solutions containing dextrose may be contraindicated in patients with known allergy to corn or corn products.
WARNINGS
Constant monitoring with an electrocardiograph is essential to the proper administration of lidocaine hydrochloride intravenously. Signs of excessive depression of cardiac conductivity, such as prolongation of the PR interval, widening of the QRS interval and the appearance or aggravation of arrhythmias, should be followed by prompt cessation of the intravenous infusion of this agent. It is mandatory to have emergency resuscitative equipment and drugs immediately available to manage adverse reactions involving cardiovascular, respiratory, or central nervous systems.
Occasional acceleration of ventricular rate may occur when lidocaine hydrochloride is administered to patients with atrial fibrillation. Evidence for proper usage in children is limited.
Anaphylactic reactions may occur following administration of lidocaine hydrochloride. (See ADVERSE REACTIONS).
In the case of severe reaction, discontinue the use of the drug.
Administer Lidocaine Hydrochloride and 5% Dextrose Injection, USP only with a calibrated infusion device.
PRECAUTIONS
General:
Caution should be employed in the repeated use of lidocaine hydrochloride in patients with severe liver or renal disease because accumulation may occur and lead to toxic phenomena, since lidocaine hydrochloride is metabolized mainly in the liver and excreted by the kidneys. The drug should also be used with caution in patients with hypovolemia and shock, and in all forms of heart block (see Contraindications and Warnings).
In patients with sinus bradycardia or incomplete heart block, the administration of lidocaine hydrochloride intravenously for the elimination of ventricular ectopic beats without prior acceleration in heart rate (e.g., by isoproterenol or by electric pacing) may promote more frequent and serious ventricular arrhythmias or complete heart block (see Contraindications).
Most potent anesthetic agents, local anesthetics of the amide type, which includes lidocaine, and muscle relaxants of both depolarizing and non-depolarizing types, have been associated with malignant hyperthermia.
Care should be taken in the administration of intravenous fluids in patients with compromised myocardial function to avoid fluid overload or disturbances of serum electrolyte concentrations which might interfere with cardiac conduction or result in congestive heart failure.
Do not administer unless solution is clear and seal is intact.
Laboratory Tests:
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations, and acid-base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation.
Drug Interactions:
Lidocaine should be used with caution in patients with digitalis toxicity accompanied by atrioventricular block (see Contraindications).
Coadministration of propranolol or cimetidine with lidocaine has been reported to reduce the clearance of lidocaine from the plasma and may result in toxic accumulation of the drug.
When lidocaine is administered with other antiarrhythmic drugs such as amiodarone, phenytoin, procainamide, propranolol or quinidine, the cardiac effects may be additive or antagonistic and toxic effects may be additive. Phenytoin may stimulate the hepatic metabolism of lidocaine, but the clinical significance of this effect is not known.
Carcinogenesis, Mutagenesis, and Impairment of Fertility:
Long term animal studies have not been performed to evaluate carcinogenic potential, mutagenic potential or the effect on fertility of lidocaine hydrochloride.
Pregnancy:
Teratogenic Effects:
Reproduction studies have been performed in rats at doses up to five times the maximum human dose and have revealed no significant findings. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, lidocaine hydrochloride should be used during pregnancy only if clearly needed.
Nursing Mothers:
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when lidocaine hydrochloride is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
Clinical studies of Lidocaine Hydrochloride did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
LIDOCAINE HYDROCHLORIDE AND DEXTROSE ADVERSE REACTIONS
Systemic reactions of the following types have been reported:
Central Nervous System: respiratory depression and arrest; unconsciousness; convulsions; tremors; twitching; vomiting; blurred or double vision; drowsiness; dizziness; light-headedness; tinnitus; sensation of heat, cold or numbness; euphoria; apprehension.
Cardiovascular System: cardiovascular arrest; bradycardia which may lead to cardiac arrest; hypotension.
Hematologic Effects: methemoglobinemia.
Allergic reactions, including anaphylactic reactions, may occur but are infrequent. There have been no reports of cross sensitivity between lidocaine hydrochloride and procainamide or between lidocaine hydrochloride and quinidine.
OVERDOSAGE
Overdosage may result in severe systemic toxicity (see Warnings and Precautions and Adverse Reactions).
LIDOCAINE HYDROCHLORIDE AND DEXTROSE DOSAGE AND ADMINISTRATION
Therapy of ventricular arrhythmias is often initiated with a single IV bolus of 50 to 100 mg of lidocaine hydrochloride injection. Following acute treatment by bolus in patients in whom arrhythmias tend to recur and who are incapable of receiving oral antiarrhythmic agents, intravenous infusion of Lidocaine Hydrochloride and 5% Dextrose Injection, USP is administered continuously at the rate of 1 to 4 mg/min (20 to 50 mcg/kg/min in the average 70 kg adult). The 0.4% solution (4 mg/mL) can be given at a rate of 15 to 60 mL/hr (0.25 to 1 mL/min). The 0.8% solution (8 mg/mL) can be given at a rate of 7.5 to 30 mL/hr (0.12 to 0.5 mL/min). Precise dose is determined by patient response.
“Pharmacokinetic data indicate reduced elimination of lidocaine after prolonged infusion (24 hours) with resultant prolongation of the half-life to approximately three times that seen following a single administration. Failure to adjust the rate of infusion in keeping with this altered ability to eliminate lidocaine may result in toxic accumulation of the drug in the patient’s serum.” LeLorier, et al., Pharmacokinetics of lidocaine after prolonged intravenous infusions in uncomplicated myocardial infarction, Ann Int. Med. 87:700-702.
Intravenous infusions of lidocaine hydrochloride must be administered under constant ECG monitoring to avoid potential overdosage and toxicity. Intravenous infusion should be terminated as soon as the patient’s basic cardiac rhythm appears to be stable or at the earliest signs of toxicity. It should rarely be necessary to continue intravenous infusions beyond 24 hours. As soon as possible and when indicated, patients should be changed to an oral antiarrhythmic agent for maintenance therapy.
Caution: When administering lidocaine hydrochloride by continuous infusion, it is advisable to closely monitor the infusion rate. Administer Lidocaine Hydrochloride and 5% Dextrose Injection, USP only with a calibrated infusion device.
Pediatric: Although controlled clinical studies to establish pediatric dosing schedules have not been conducted, the American Heart Association’s Standards and Guidelines recommends a bolus dose of 1 mg/kg followed by an infusion rate of 30 µg/kg/min. American Heart Association, Standards and Guidelines for Cardiopulmonary Resuscitation (CPR) and Emergency Cardiac Care (ECC), JAMA Vol. 244. No. 5: 453-509.
Lidocaine hydrochloride should not be added to blood transfusion assemblies.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Use of a final filter is recommended during administration of all parenteral solutions, where possible.
All injections in VIAFLEX Plus plastic containers are intended for intravenous administration using sterile equipment.
Because dosages of this drug are titrated to response, no additives should be made to Lidocaine Hydrochloride and 5% Dextrose Injection, USP.
HOW SUPPLIED
Lidocaine Hydrochloride and 5% Dextrose Injection, USP in VIAFLEX plastic container is available as follows:
| Code | Size (mL) | NDC | Product Name |
| 2B0972 | 250 | 0338-0409-02 | Lidocaine Hydrochloride and 5% Dextrose |
| 2B0973 | 500 | 0338-0409-03 | Injection, USP (4 mg/mL) |
| 2B0962 | 250 | 0338-0411-02 | Lidocaine Hydrochloride and 5% Dextrose Injection, USP (8 mg/mL) |
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. It is recommended the product be stored at room temperature (25°C); brief exposure up to 40°C does not adversely affect the product.
DIRECTIONS FOR USE OF VIAFLEX PLUS PLASTIC CONTAINER
WARNING: Do not use plastic containers in series connections. Such use could result in air embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is completed.
To Open:
Tear overwrap down side at slit and remove solution container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually. Check for minute leaks by squeezing inner bag firmly. If leaks are found, discard solution as sterility may be impaired. Do not add supplementary medication.
Preparation for Administration:
- Suspend container from eyelet support.
- Remove protector from outlet port at bottom of container.
- Attach administration set. Refer to complete directions accompanying set.
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Printed in USA
*Bar Code Position Only
071968966
07-19-68-966
Rev. April 2012
BAXTER, VIAFLEX, and PL 146 are trademarks of Baxter International Inc.

LOT
EXP
NDC 0338-0411-02
Lidocaine
Lidocaine Hyrdrochloride and
5% Dextrose Injection USP
2g
per 250 mL
(8 mg/mL)
250 mL STERILE SINGLE
DOSE CONTAINER
EACH
100 mL CONTAINS 800 mg
LIDOCAINE HCI USP AND 5 g
DEXTROSE HYDROUS USP pH
4.0 (3.0 TO 7.0) OSMOLARITY
311 mOsmol/L (CALC) USUAL
DOSAGE SEE INSERT
DO
NOT ADD SUPPLEMENTARY
MEDICATION FOR USE ONLY WITH
A CALIBRATED INFUSION DEVICE
STORE IN MOISTURE BARRIER
OVERWRAP AT ROOM TEMPERATURE
(77°F OR 25°C) UNTIL READY TO
USE RX ONLY
Baxter
USA 2B0962
50
100
150
200

Lot: PXXXXXX
QTY: 24-250mL
Exp: XXXXX
Code: 2B0962
NDC: 0338-0411-02
LIDOCAINE HYDROCHLORIDE
AND 5% DEXTROSE INJECTION
, USP
2g per 250 mL
(17)XXXXX00 (10) PXXXXXX
(01) 50303380411028
01/09/13 12:11:41
Lidocaine Hydrochloride and DextroseLidocaine Hydrochloride INJECTION, SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Lidocaine Hydrochloride and DextroseLidocaine Hydrochloride INJECTION, SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||