Levothyroxine Sodium
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- LEVOTHYROXINE SODIUM DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- INDICATIONS & USAGE
- LEVOTHYROXINE SODIUM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- PEDIATRIC USE
- GERIATRIC USE
- LEVOTHYROXINE SODIUM ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
Thyroid hormones, including Levothyroxine sodium tablets, USP, either alone or with other therapeutic agents, should not be used for the treatment of obesity or for weight loss. In euthyroid patients, doses within the range of daily hormonal requirements are ineffective for weight reduction. Larger doses may produce serious or even life threatening manifestations of toxicity, particularly when given in association with sympathomimetic amines such as those used for their anorectic effects.LEVOTHYROXINE SODIUM DESCRIPTION
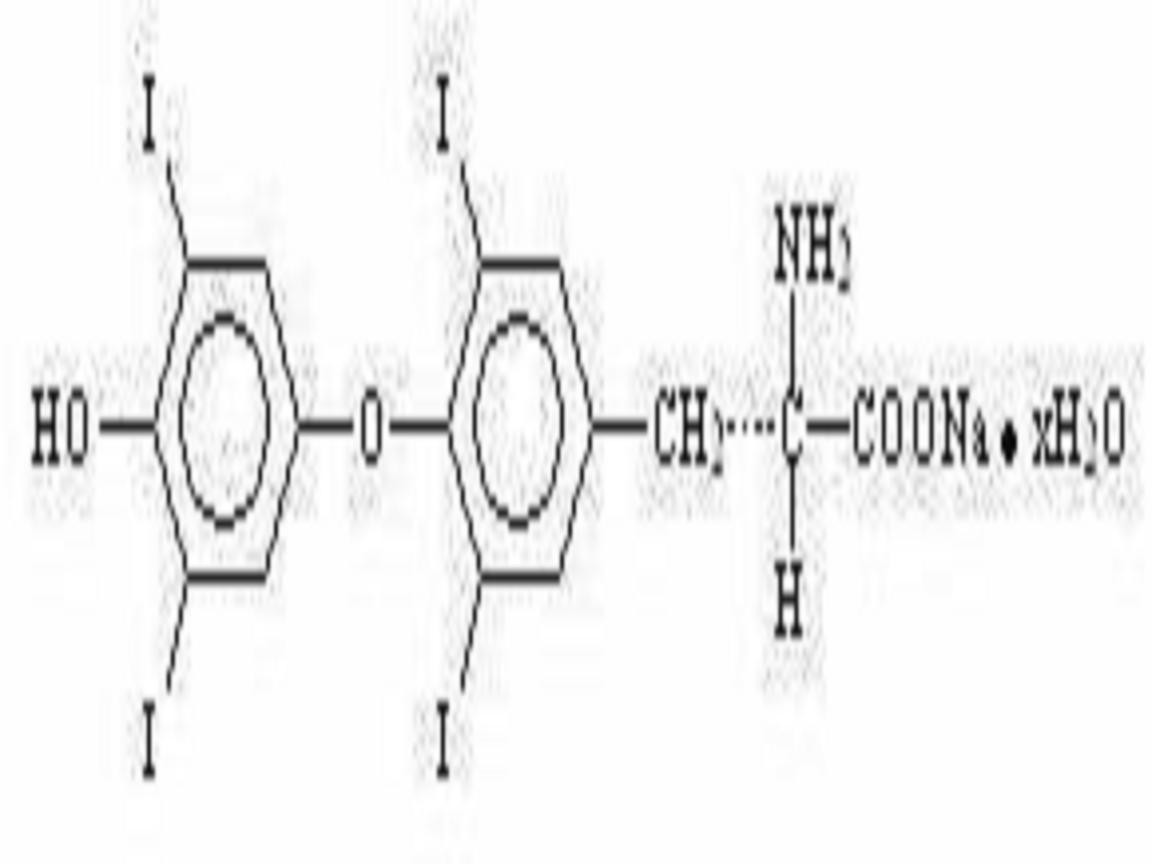
Inactive Ingredients
CLINICAL PHARMACOLOGY
INDICATIONS AND USAGEPRECAUTIONSDOSAGE AND ADMINISTRATION
PHARMACOKINETICS
AbsorptionPRECAUTIONS, Drug InteractionsDrug-Food InteractionsDistributionPRECAUTIONS, Drug InteractionsDrug-Laboratory Test InteractionsPRECAUTIONS, Pregnancy
MetabolismTable 1
Elimination
INDICATIONS & USAGE
Hypothyroidism
Pituitary TSH SuppressionWARNINGSPRECAUTIONSWARNINGSPRECAUTIONSWARNINGSPRECAUTIONS
LEVOTHYROXINE SODIUM CONTRAINDICATIONS
PRECAUTIONSDESCRIPTION, Inactive IngredientsWARNINGS
Thyroid hormones, including Levothyroxine sodium tablets, USP, either alone or with other therapeutic agents, should not be used for the treatment of obesity or for weight loss. In euthyroid patients, doses within the range of daily hormonal requirements are ineffective for weight reduction. Larger doses may produce serious or even life threatening manifestations of toxicity, particularly when given in association with sympathomimetic amines such as those used for their anorectic effects.CONTRAINDICATIONS
PRECAUTIONS
GeneralDrug Interactions
Effects on bone mineral density
Patients with underlying cardiovascular diseaseWARNINGSPRECAUTIONS, Geriatric Use, andDOSAGE AND ADMINISTRATION). If cardiac symptoms develop or worsen, the levothyroxine dose should be reduced or withheld for one week and then cautiously restarted at a lower dose. Overtreatment with levothyroxine sodium may have adverse cardiovascular effects such as an increase in heart rate, cardiac wall thickness, and cardiac contractility and may precipitate angina or arrhythmias. Patients with coronary artery disease who are receiving levothyroxine therapy should be monitored closely during surgical procedures, since the possibility of precipitating cardiac arrhythmias may be greater in those treated with levothyroxine. Concomitant administration of levothyroxine and sympathomimetic agents to patients with coronary artery disease may precipitate coronary insufficiency.
Patients with nontoxic diffuse goiter or nodular thyroid disease-Exercise caution when administering levothyroxine to patients with nontoxic diffuse goiter or nodular thyroid disease in order to prevent precipitation of thyrotoxicosis (seeWARNINGS). If the serum TSH is already suppressed, levothyroxine sodium should not be administered (seeCONTRAINDICATIONS).
Associated endocrine disorders
Hypothalamic/pituitary hormone deficiencies-In patients with secondary or tertiary hypothyroidism, additional hypothalamic/pituitary hormone deficiencies should be considered, and, if diagnosed, treated (seePRECAUTIONS, Autoimmune polyglandular syndromefor adrenal insufficiency).
Autoimmune polyglandular syndrome
Occasionally, chronic autoimmune thyroiditis may occur in association with other autoimmune disorders such as adrenal insufficiency, pernicious anemia, and insulin-dependent diabetes mellitus. Patients with concomitant adrenal insufficiency should be treated with replacement glucocorticoids prior to initiation of treatment with levothyroxine sodium. Failure to do so may precipitate an acute adrenal crisis when thyroid hormone therapy is initiated, due to increased metabolic clearance of glucocorticoids by thyroid hormone. Patients with diabetes mellitus may require upward adjustments of their antidiabetic therapeutic regimens when treated with levothyroxine (seePRECAUTIONS, Drug Interactions).
Other associated medical conditions
Infants with congenital hypothyroidism appear to be at increased risk for other congenital anomalies, with cardiovascular anomalies (pulmonary stenosis, atrial septal defect, and ventricular septal defect) being the most common association.
INFORMATION FOR PATIENTS
LABORATORY TESTS
PRECAUTIONS, Drug InteractionsDrug-Laboratory Test Interactions
WARNINGSPRECAUTIONSDOSAGE AND ADMINISTRATION
PRECAUTIONS, Pediatric UseDOSAGE AND ADMINISTRATION
DRUG INTERACTIONS
Table 2
Table 2
Table 2
PEDIATRIC USE
DOSAGE AND ADMINISTRATIONTable 3PRECAUTIONS, Laboratory Tests
PRECAUTIONS
PRECAUTIONS, Laboratory TestsDOSAGE and ADMINISTRATION
GERIATRIC USE
WARNINGSPRECAUTIONSDOSAGE AND ADMINISTRATIONLEVOTHYROXINE SODIUM ADVERSE REACTIONS
PRECAUTIONSOVERDOSAGEGeneral:
Central nervous system:
Musculoskeletal:
Cardiovascular:
Respiratory:
Gastrointestinal:
Dermatologic:
Endocrine:
Reproductive:
OVERDOSAGE
PRECAUTIONSADVERSE REACTIONSTreatment of Overdosage
Acute Massive Overdosage
DOSAGE & ADMINISTRATION
General PrinciplesWARNINGSPRECAUTIONSPRECAUTIONS, Laboratory Tests
PRECAUTIONS, Drug InteractionsInformation for Patients
PRECAUTIONS
Specific Patient Populations
WARNINGSPRECAUTIONS, Laboratory Tests
PRECAUTIONS, Laboratory Tests
PRECAUTIONS, Pediatric Use
PRECAUTIONS , Drug-Food Interactions
Table 3
PRECAUTIONS, Laboratory TestsPediatric UsePregnancy
CONTRAINDICATIONSWARNINGSPRECAUTIONS
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Levothyroxine SodiumLevothyroxine Sodium TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!