LBel
L'BELSUPRÉMACIE TEINT
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Purpose
- LBel Uses
- Warnings
- Directions
- LBel Other information
- Inactive ingredients
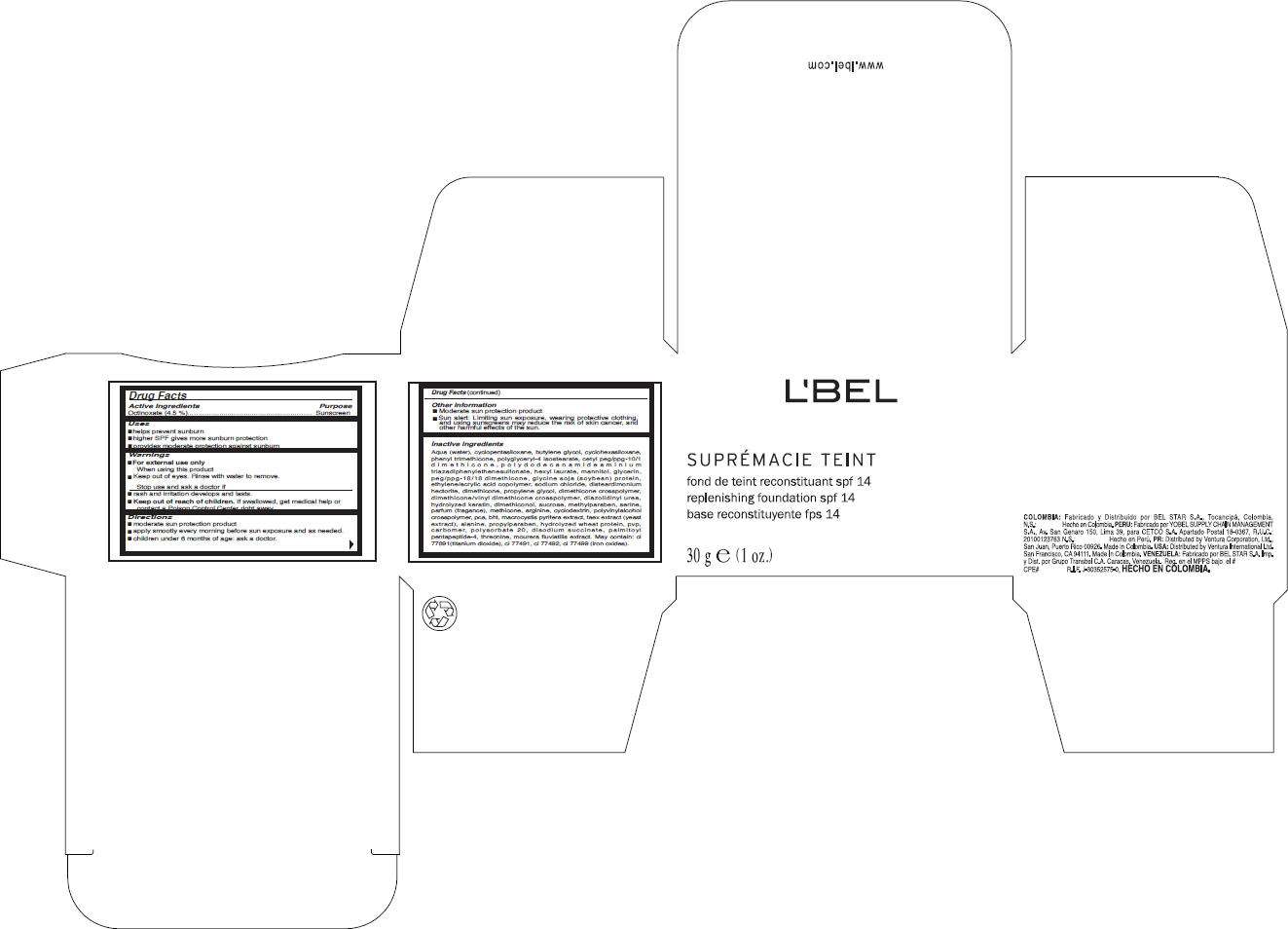
- PRINCIPAL DISPLAY PANEL - 30 g Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredients
Octinoxate (4.5 %)
Purpose
Sunscreen
LBel Uses
- helps prevent sunburn
- higher SPF gives more sunburn protection
- provides moderate protection against sunburn
Warnings
- For external use only.
When using this product
- keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
- rash and irritation develops and lasts.
- Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- moderate sun protection product
- apply smootly every morning before sun exposure and as needed.
- children under 6 months of age: ask a doctor.
LBel Other information
- Sun alert: Limiting sun exposure, wearing protective clothing, and using sunscreens may reduce the risk of skin cancer, and other harmful effects of the sun.
Inactive ingredients
Aqua (water), cyclopentasiloxane, butylene glycol, cyclohexasiloxane, phenyl trimethicone, polyglyceryl-4 isostearate, cetyl peg/ppg-10/1 dimethicone, polydodecanamideaminium triazadiphenylethenesulfonate, hexyl laurate, mannitol, glycerin, peg/ppg-18/18 dimethicone, glycine soja (soybean) protein, ethylene/acrylic acid copolymer, sodium chloride, disteardimonium hectorite, dimethicone, propylene glycol, dimethicone crosspolymer, dimethicone/vinyl dimethicone crosspolymer, diazolidinyl urea, hydrolyzed keratin, dimethiconol, sucrose, methylparaben, serine, parfum (fragance), methicone, arginine, cyclodextrin, polyvinylalcohol crosspolymer, pca, bht, macrocystis pyrifera extract, faex extract (yeast extract), alanine, propylparaben, hydrolyzed wheat protein, pvp, carbomer, polysorbate 20, disodium succinate, palmitoyl pentapeptide-4, threonine, mourera fluviatilis extract. May contain: ci 77891(titanium dioxide), ci 77491, ci 77492, ci 77499 (iron oxides).
US: Distributed by Ventura International, Ltd. San Francisco, CA 94111
PRINCIPAL DISPLAY PANEL - 30 g Carton
L'BEL
SUPRÉMACIE TEINT
replenhising foundation spf 14
30 g e (1 oz.)
LBelOctinoxate CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||