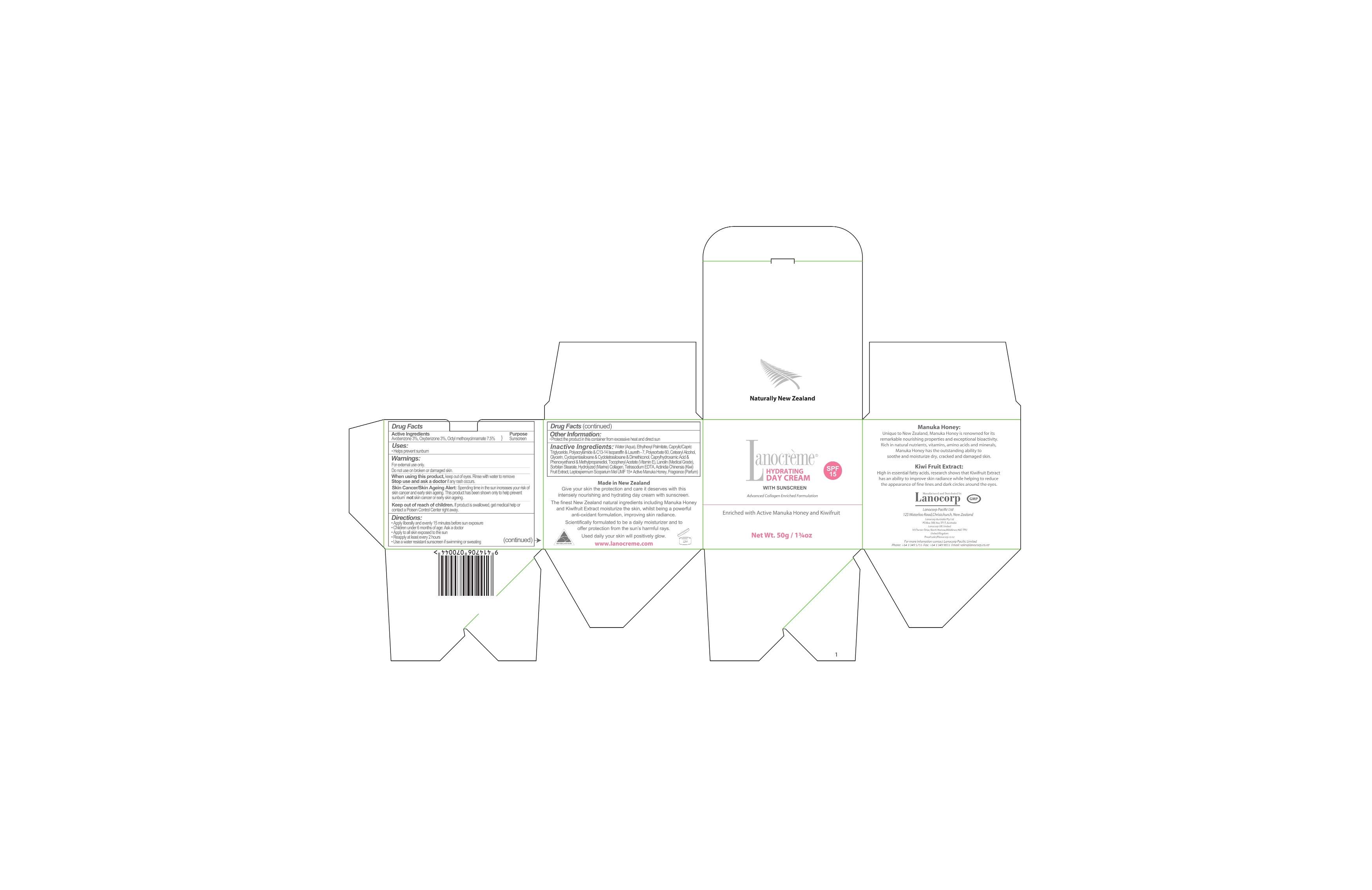
LANOCREME HYDRATING
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
ACTIVE INGREDIENTS
Avobenzone 3%
Oxybenzone 3%
Octylmethoxycinnamate 7.5%
DIRECTIONS
Apply liberally and evenly 15 minutes before sun exposure
Children under 6 months of age: Ask a doctor
Apply to all skin exposed to the sun
Reapply at least every 2 hours
Use a water resistant sunscreen if swimming or sweating
WARNINGS
For external use only. Do not use on broken or damaged skin.
Skin Cancer/Skin Ageing Alert: Spending time in the sun increases your risk of skin cancer and early skin ageing. This product has been shown only to help prevent sunburn, not skin cancer or early skin ageing.
Keep out of reach of children.
Keep out of reach of children. If product is swalowed, get medical help or contact a Poison Control Center right away.
When using this product, keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if any rush occurs.
INACTIVE INGREDIENTS
Water
Ethylhexyl palmitate
Caprylic triglyceride
Capric triglyceride
Polyacrylamide
c13-14 Isoparaffin
Laureth-7
Polysorbate 60
Cetearyl alcohol
Glycerin
Cyclopentasiloxane
Clyclotetrasiloxane
dimethiconol
Caprylhydroxamic acid
phenoxyethanol
methylpropanediol
tocopheryl acetate (vitamin e)
lanolin (medical grade)
Sorbitan stearate
Hydrolyzed (Marine) collagen
Tetrasodium EDTA
Actinidia chinensis (kiwi) fruit extract
Leptospermum scoparium Mel UMF 15+ Active Manuka Honey

LANOCREME HYDRATINGAVOBENZONE, OXYBENZONE, OCTYL METHOXYCINNAMATE CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||