Laneige Sliding Pact EX
LANEIGE SLIDING PACT_EX SNOW CRYSTAL SPF25 PA++
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACTIVE INGREDIENTS
- PURPOSE
- USE
- WARNINGS
- DIRECTIONS
- INACTIVE INGREDIENTS
- OTHER INFORMATION
- PRINCIPAL DISPLAY PANEL - 12 g Container Carton, No. 1
- PRINCIPAL DISPLAY PANEL - 12 g Container Carton, No. 2
- PRINCIPAL DISPLAY PANEL - 12 g Container Carton, No. 3
- PRINCIPAL DISPLAY PANEL - 12 g Container Carton, No. 4
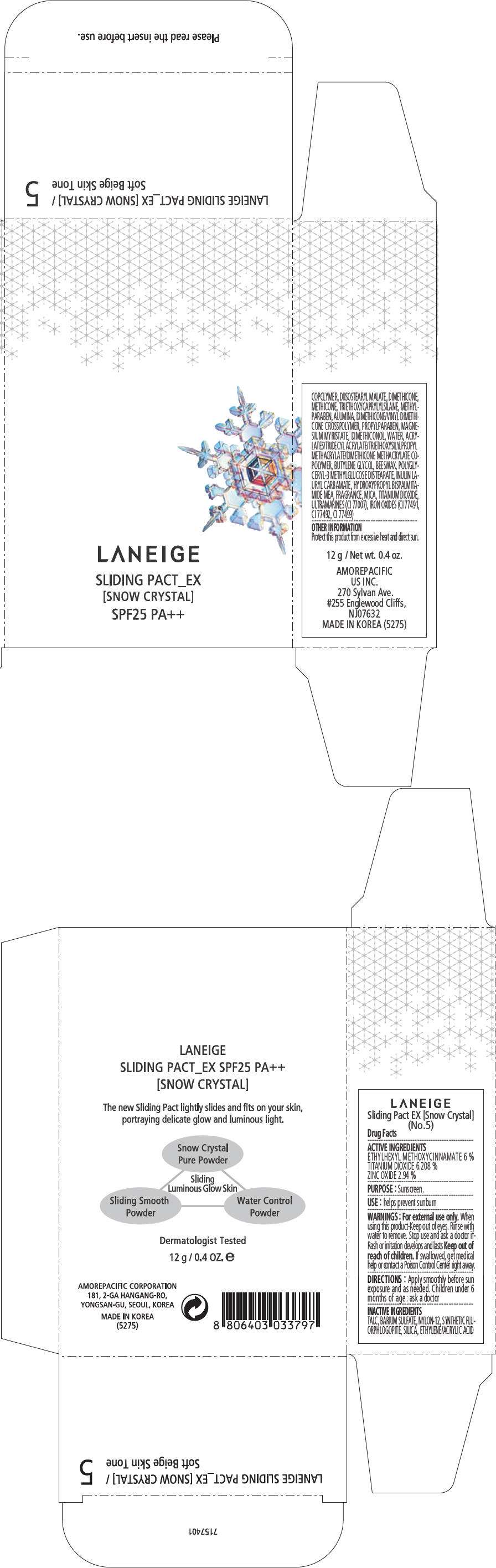
- PRINCIPAL DISPLAY PANEL - 12 g Container Carton, No. 5
FULL PRESCRIBING INFORMATION
Drug Facts
ACTIVE INGREDIENTS
ETHYLHEXYL METYHOXYCINNAMATE 6 %
TITANIUM DIOXIDE 6.208 %
ZINC OXIDE 2.94 %
PURPOSE
Sunscreen.
USE
helps prevent sunburn
WARNINGS
For external use only.
When using this product-Keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if-Rash or irritation develops and lasts
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
Apply smoothly before sun exposure and as needed. Children under 6 months of age: ask a doctor
INACTIVE INGREDIENTS
TALC, BARIUM SULFATE, NYLON-12, SYNTHETIC FLUORPHLOGOPITE, SILICA, ETHYLENE/ACRYLIC ACID COPOLYMER, DIISOSTEARYL MALATE, DIMETHICONE, METHICONE, TRIETHOXYCAPRYLYLSILANE, METHYLPARABEN, ALUMINA, DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER, PROPYLPARABEN, MAGNESIUM MYRISTATE, DIMETHICONOL, WATER, ACRYLATES/TRIDECYL ACRYLATE/TRIETHOXYSILYLPROPYL METHACRYLATE/DIMETHICONE METHACRYLATE COPOLYMER, BUTYLENE GLYCOL, BEESWAX, POLYGLYCERYL-3 METHYLGLUCOSE DISTEARATE, INULIN LAURYL CARBAMATE, HYDROXYPROPYL BISPALMITAMIDE MEA, FRAGRANCE, MICA, TITANIUM DIOXIDE, ULTRAMARINES (CI 77007), IRON OXIDES (CI 77491, CI 77492, CI 77499)
OTHER INFORMATION
Protect this product from excessive heat and direct sun.
AMOREPACIFIC US INC.
270 Sylvan Ave.
#255 Englewood Cliffs, NJ07632
PRINCIPAL DISPLAY PANEL - 12 g Container Carton, No. 1
LANEIGE
SLIDING PACT_EX
[SNOW CRYSTAL]
SPF25 PA++
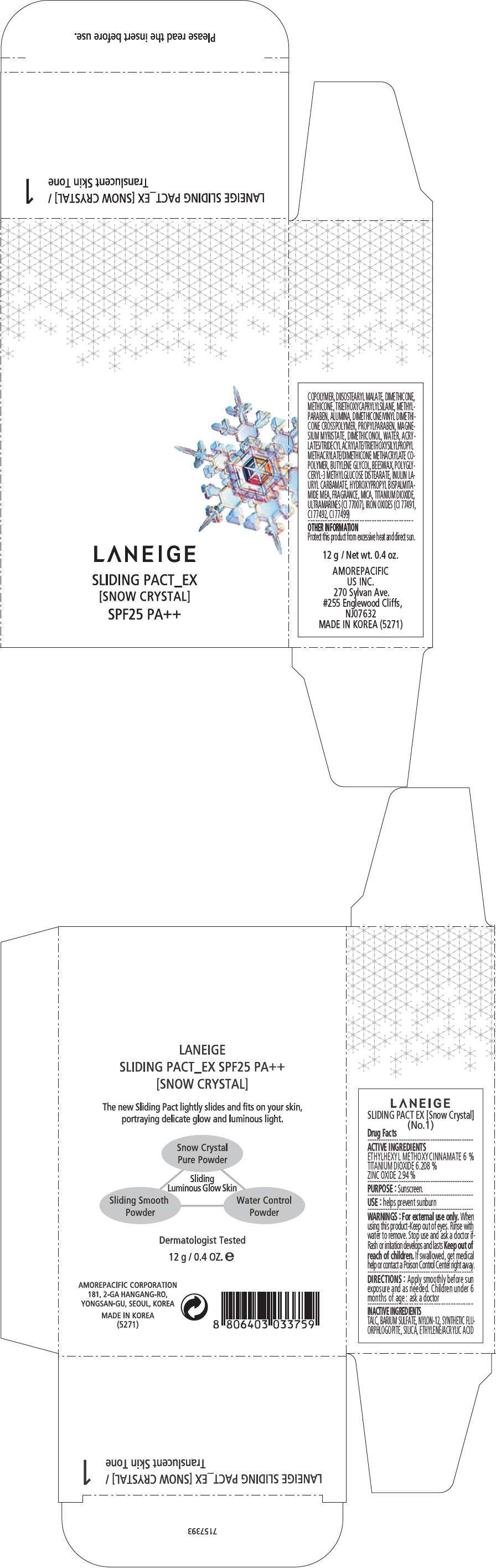
PRINCIPAL DISPLAY PANEL - 12 g Container Carton, No. 2
LANEIGE
SLIDING PACT_EX
[SNOW CRYSTAL]
SPF25 PA++
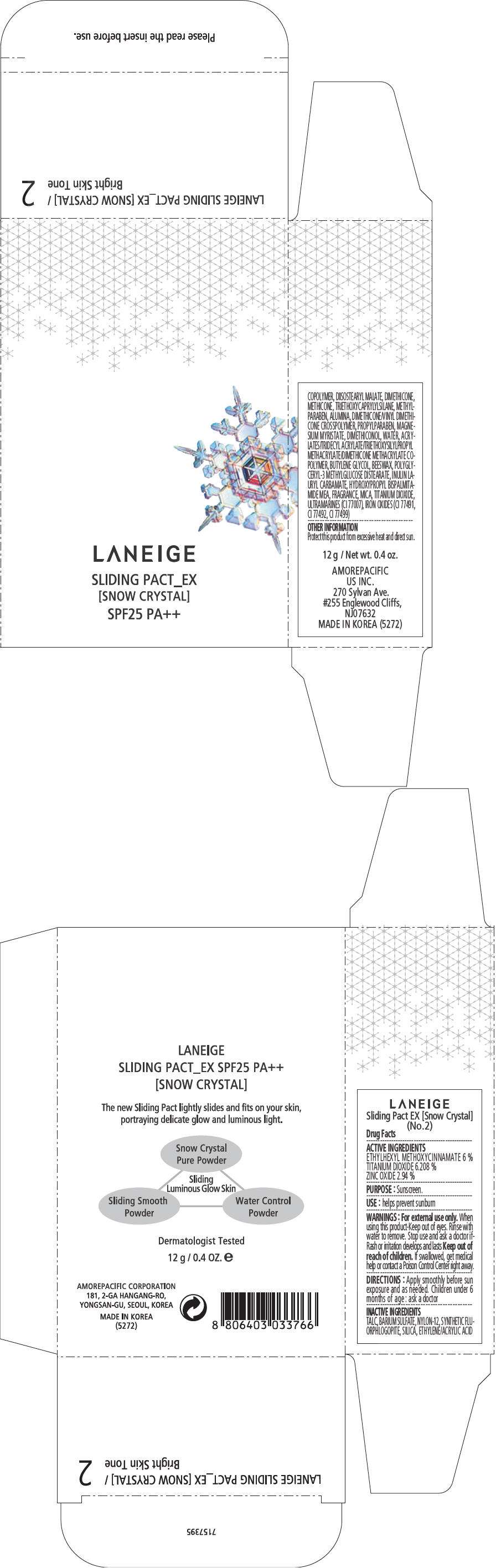
PRINCIPAL DISPLAY PANEL - 12 g Container Carton, No. 3
LANEIGE
SLIDING PACT_EX
[SNOW CRYSTAL]
SPF25 PA++
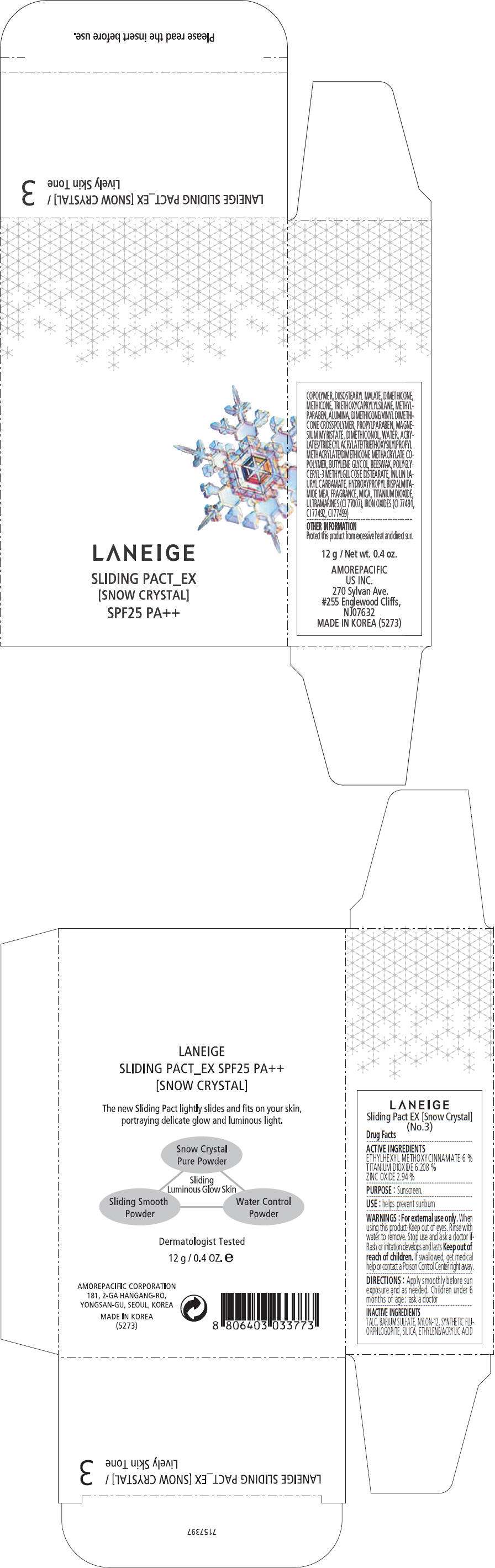
PRINCIPAL DISPLAY PANEL - 12 g Container Carton, No. 4
LANEIGE
SLIDING PACT_EX
[SNOW CRYSTAL]
SPF25 PA++
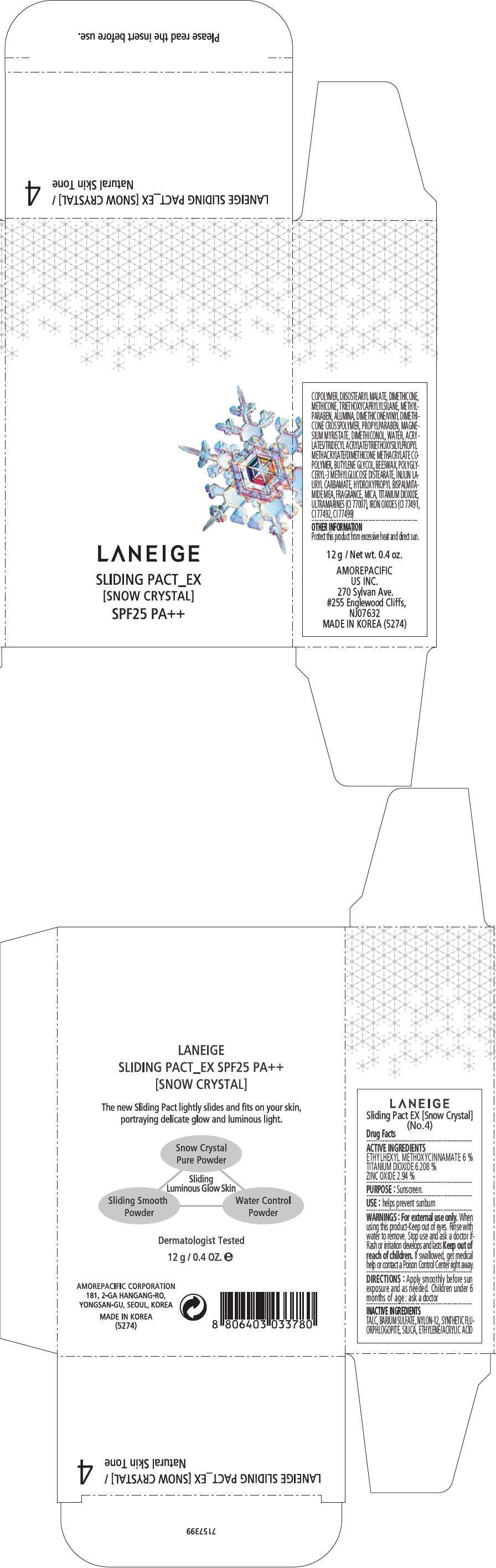
PRINCIPAL DISPLAY PANEL - 12 g Container Carton, No. 5
LANEIGE
SLIDING PACT_EX
[SNOW CRYSTAL]
SPF25 PA++
Laneige Sliding Pact EXOCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Laneige sliding pact EXOCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Laneige sliding pact EXOCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Laneige sliding pact EXOCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Laneige sliding pact EXOCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||