Kleenex Ultra Moisturizing Foam Hand Sanitizer
Kleenex Ultra Moisturizing Foam Hand Sanitizer
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Use
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- Questions?
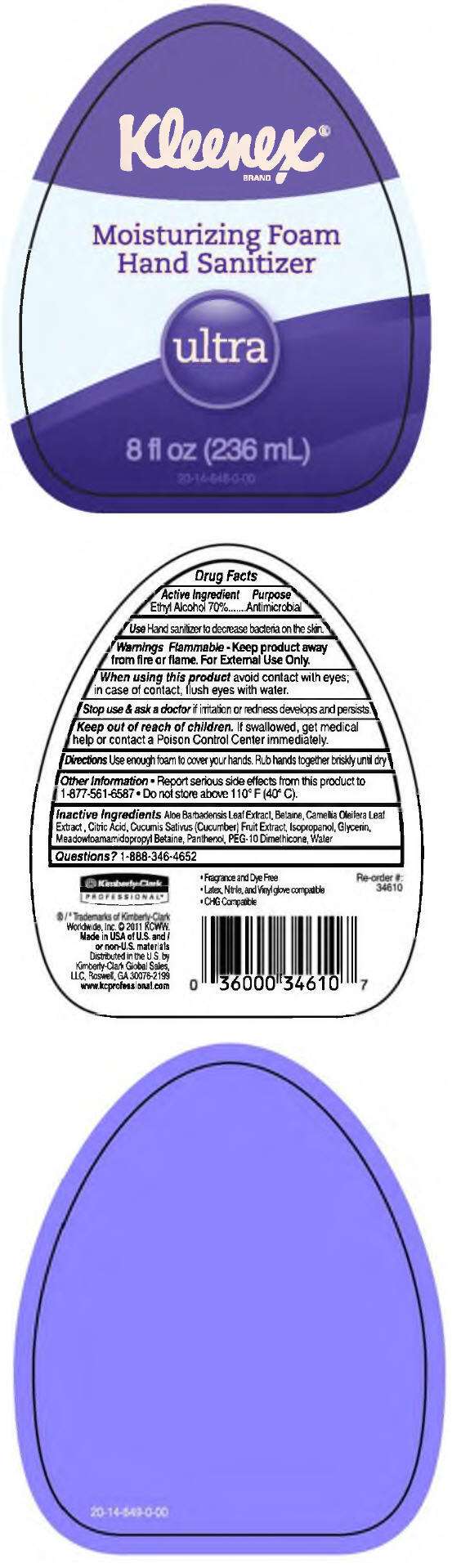
- PRINCIPAL DISPLAY PANEL - 236 mL Bottle Label
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredient
Ethyl Alcohol 70%
Purpose
Antimicrobial
Use
Hand sanitizer to decrease bacteria on the skin.
Warnings
Flammable - Keep product away from fire or flame. For External Use Only.
When using this product avoid contact with eyes; in case of contact, flush eyes with water.
Stop use & ask a doctor if irritation or redness develops and persists.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
Use enough foam to cover your hands. Rub hands together briskly until dry.
Other Information
- Report serious side effects from this product to 1-877-561-6587
- Do not store above 110° F (40° C).
Inactive Ingredients
Aloe Barbadensis Leaf Extract, Betaine, Camellia Oleifera Leaf Extract, Citric Acid, Cucumis Sativus (Cucumber) Fruit Extract, Isopropanol, Glycerin, Meadowfoamamidopropyl Betaine, Panthenol, PEG-10 Dimethicone, Water
Questions?
1-888-346-4652
Distributed in the U.S. by
Kimberly-Clark Global Sales,
LLC, Roswell, GA 30076-2199
PRINCIPAL DISPLAY PANEL - 236 mL Bottle Label
Kleenex
®
BRAND
Moisturizing Foam
Hand Sanitizer
ultra
8 fl oz (236 mL)
20-14-545-0-00
Kleenex Ultra Moisturizing Foam Hand SanitizerAlcohol SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||