Ketoconazole
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- KETOCONAZOLE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- KETOCONAZOLE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- KETOCONAZOLE ADVERSE REACTIONS
- OVERDOSAGE
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
WARNINGWARNINGSPRECAUTIONS
CONTRAINDICATIONSWARNINGSPRECAUTIONS
CONTRAINDICATIONSWARNINGSPRECAUTIONS
CONTRAINDICATIONSWARNINGSPRECAUTIONS
KETOCONAZOLE DESCRIPTION
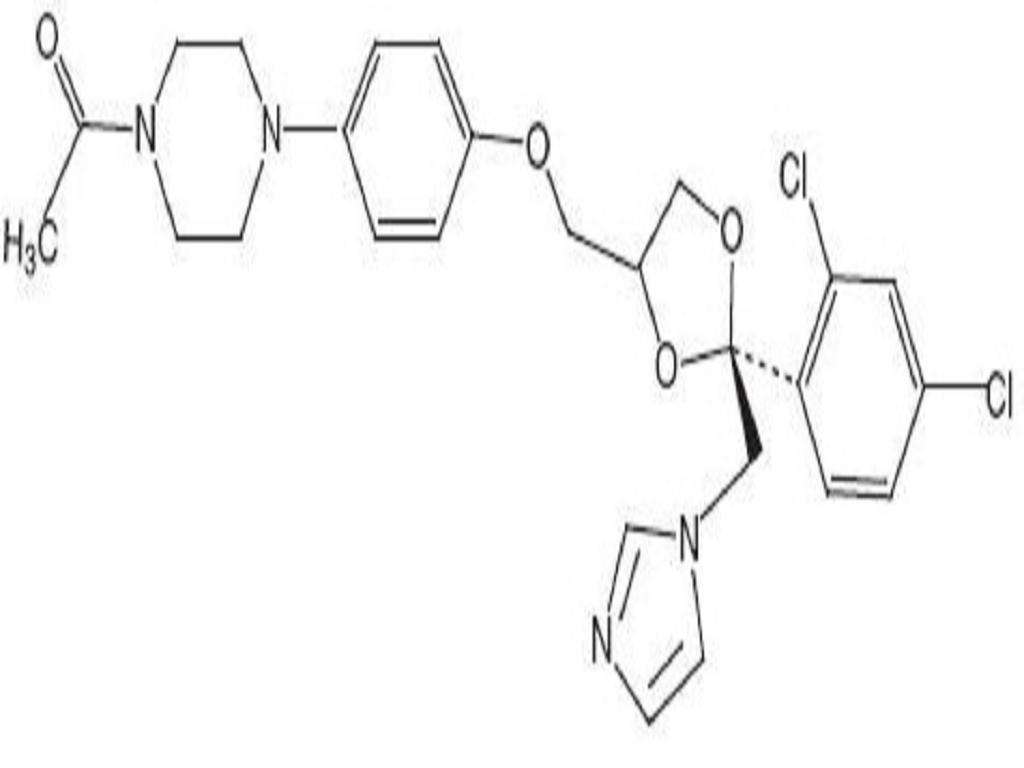
CLINICAL PHARMACOLOGY
Mode of Action
INDICATIONS & USAGE
KETOCONAZOLE CONTRAINDICATIONS
BOX WARNINGWARNINGSPRECAUTIONSBOX WARNINGWARNINGSPRECAUTIONS
PRECAUTIONS
WARNINGS
Hepatotoxicity, primarily of the hepatocellular type, has been associated with the use of ketoconazole tablets, including rare fatalities. The reported incidence of hepatotoxicity has been about 1:10,000 exposed patients, but this probably represents some degree of under-reporting, as is the case for most reported adverse reactions to drugs. The median duration of ketoconazole tablet therapy in patients who developed symptomatic hepatotoxicity was about 28 days, although the range extended to as low as 3 days. The hepatic injury has usually, but not always, been reversible upon discontinuation of ketoconazole tablet treatment. Several cases of hepatitis have been reported in children.BOX WARNINGCONTRAINDICATIONSPRECAUTIONS
BOX WARNINGCONTRAINDICATIONSPRECAUTIONS
PRECAUTIONS
GeneralINFORMATION FOR PATIENTS
Patients should be instructed to report any signs and symptoms which may suggest liver dysfunction so that appropriate biochemical testing can be done. Such signs and symptoms may include unusual fatigue, anorexia, nausea and/or vomiting, jaundice, dark urine or pale stools (seeWARNINGSsection).DRUG INTERACTIONS
BOX WARNINGCONTRAINDICATIONSWARNINGS
BOX WARNINGCONTRAINDICATIONSWARNINGS
BOX WARNINGCONTRAINDICATIONSWARNINGS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic Effects. Pregnancy Category CNonteratogenic Effects
NURSING MOTHERS
PEDIATRIC USE
KETOCONAZOLE ADVERSE REACTIONS
In rare cases, anaphylaxis has been reported after the first doseWARNINGSBOX WARNINGCONTRAINDICATIONSWARNINGSCONTRAINDICATIONSWARNINGSPRECAUTIONS
OVERDOSAGE
AdultsChildren
HOW SUPPLIED
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

KetoconazoleKetoconazole TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!