Isosorbide Dinitrate
FULL PRESCRIBING INFORMATION: CONTENTS*
- ISOSORBIDE DINITRATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- ISOSORBIDE DINITRATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- ISOSORBIDE DINITRATE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
ISOSORBIDE DINITRATE DESCRIPTION
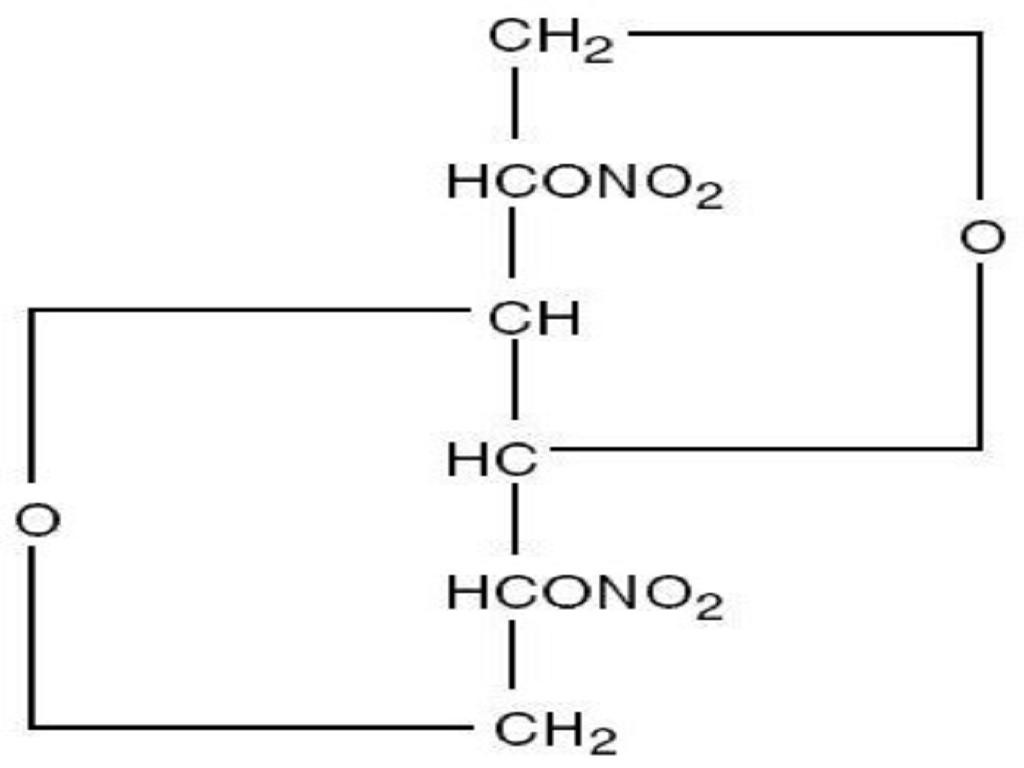
CLINICAL PHARMACOLOGY
Pharmacokinetics
Clinical Trials
INDICATIONS & USAGE
ISOSORBIDE DINITRATE CONTRAINDICATIONS
WARNINGS
Amplification of the vasodilatory effects of isosorbide dinitrate by sildenafil can result in severe hypotension. The time course and dose dependence of this interaction have not been studied. Appropriate supportive care has not been studied, but it seems reasonable to treat this as a nitrate overdose, with elevation of the extremities and with central volume expansion.PRECAUTIONS
GeneralINFORMATION FOR PATIENTS
DRUG INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Category CNURSING MOTHERS
PEDIATRIC USE
ISOSORBIDE DINITRATE ADVERSE REACTIONS
OVERDOSAGE
OVERDOSAGE
Hemodynamic EffectsMethemoglobinemia
DOSAGE & ADMINISTRATION
CLINICAL PHARMACOLOGYCLINICAL PHARMACOLOGY
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Isosorbide DinitrateIsosorbide Dinitrate TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!