Irbesartan
Camber Pharmaceuticals, Inc.
Hetero Labs Limited Unit V
Irbesartan Tablets USP Rx only
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING: FETAL TOXICITY
- IRBESARTAN DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- INDICATIONS & USAGE
- IRBESARTAN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- Impaired Renal Function
- Information for Patients
- Drug Interactions
- Non-Steroidal Anti-Inflammatory Agents Including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)
- Dual Blockade of the Renin-Angiotensin System (RAS)
- Carcinogenesis & Mutagenesis & Impairment Of Fertility
- Pregnancy
- Nursing Mothers
- Pediatric Use
- Geriatric Use
- IRBESARTAN ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- SPL PATIENT PACKAGE INSERT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
WARNING: FETAL TOXICITY
• When pregnancy is detected, discontinue irbesartan as soon as possible.
• Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. See WARNINGS: Fetal Toxicity.
IRBESARTAN DESCRIPTION
25286
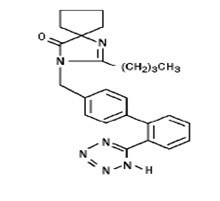
CLINICAL PHARMACOLOGY
Mechanism of Action
1 2
1 12
1
Pharmacokinetics
Metabolism and Elimination
14
14
In vitro
Distribution
1
Special Populations
Gender
Geriatric
max
Race
max
Renal Insufficiency
WARNINGS: Hypotension in Volume- or Salt-Depleted Patients DOSAGE AND ADMINISTRATION
Hepatic Insufficiency
Drug Interactions
PRECAUTIONSDrug Interactions
Pharmacodynamics
CLINICAL STUDIES
Hypertension
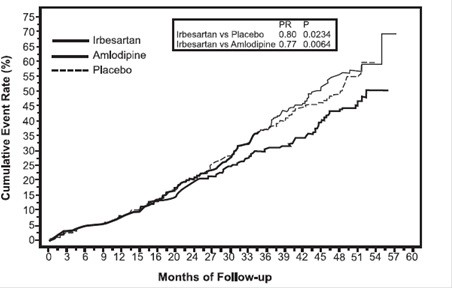
Nephropathy in Type 2 Diabetic Patients
Figure 3
IDNT: Kaplan-Meier Estimates Of Primary Endpoint
(Doubling of Serum Creatinine, End-Stage Renal Disease or All-Cause Mortality)
|
Table 1: IDNT: Components of Primary Composite Endpoint
|
|||||||
|
Irbesartan N=579
(%) |
Comparison With Placebo
|
Comparison With Amlodipine
|
|||||
|
Placebo
N=569 (%) |
Hazard
Ratio |
95% CI
|
Amlodipine
N=567 (%) |
Hazard Ratio |
95% CI
|
||
|
Primary Composite Endpoint |
32.6
|
39.0
|
0.80
|
0.66-0.97(p=0.0234) |
41.1 |
0.77
|
0.63-0.93 |
| Breakdown of first occurring event contributing to primary endpoint |
|||||||
| 2x creatinine
|
14.2
|
19.5
|
---
|
---
|
22.8
|
---
|
--- |
| ESRD
|
7.4
|
8.3
|
---
|
---
|
8.8
|
---
|
---
|
| Death
|
11.1
|
11.2
|
---
|
---
|
9.5
|
---
|
---
|
| Incidence of total events over entire period of follow-up |
|||||||
| 2x creatinine
|
16.9
|
23.7
|
0.67
|
0.52-0.87
|
25.4
|
0.63
|
0.49-0.81
|
| ESRD
|
14.2
|
17.8
|
0.77
|
0.57-1.03
|
18.3
|
0.77
|
0.57-1.03
|
| Death |
15.0 |
16.3 |
0.92 |
0.69-1.23 |
14.6 |
1.04 |
0.77-1.40 |
The secondary endpoint of the study was a composite of cardiovascular mortality and morbidity (myocardial infarction, hospitalization for heart failure, stroke with permanent neurological deficit, amputation). There were no statistically significant differences among treatment groups in these endpoints. Compared with placebo, irbesartan significantly reduced proteinuria by about 27%, an effect that was evident within 3 months of starting therapy. Irbesartan significantly reduced the rate of loss of renal function (glomerular filtration rate), as measured by the reciprocal of the serum creatinine concentration, by 18.2%.
|
Table 2: IDNT: Primary Efficacy Outcome Within Subgroups |
||||
|
Baseline Factors |
Irbesartan N=579 (%) |
Comparison With Placebo |
||
|
Placebo N=569 (%) |
Hazard Ratio |
95% Cl |
||
|
Gender |
||||
|
Male |
27.5 |
36.7 |
0.68 |
0.53-0.88 |
|
Female |
42.3 |
44.6 |
0.98 |
0.72-1.34 |
|
Race |
||||
|
White |
29.5 |
37.3 |
0.75 |
0.60-0.95 |
|
Non-White |
42.6 |
43.5 |
0.95 |
0.67-1.34 |
|
Age (years) |
|
|
|
|
|
< 65 |
31.8 |
39.9 |
0.77 |
0.62-0.97 |
|
≥ 65 |
35.1 |
36.8 |
0.88 |
0.61-1.29 |
INDICATIONS & USAGE
Hypertension
Nephropathy in Type 2 Diabetic Patients
CLINICAL PHARMACOLOGY:Clinical Studies
IRBESARTAN CONTRAINDICATIONS
PRECAUTIONSDrug Interactions
WARNINGS
Fetal Toxicity
Pregnancy Category D
PRECAUTIONSPediatric Use
Hypotension in Volume- or Salt-Depleted Patients
DOSAGE AND ADMINISTRATION
PRECAUTIONS
Impaired Renal Function
Information for Patients
Pregnancy
Drug Interactions
In vitroin vitro
Non-Steroidal Anti-Inflammatory Agents Including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)
Dual Blockade of the Renin-Angiotensin System (RAS)
Carcinogenesis & Mutagenesis & Impairment Of Fertility
0 to 24 hour
in vitroin vitroin vivo
0 to 24 hour
Pregnancy
Teratogenic Effects:
Pregnancy Category D
WARNINGS: Fetal Toxicity.
Nursing Mothers
Pediatric Use
Neonates with a history of in utero exposure to irbesartan:
Geriatric Use
CLINICAL PHARMACOLOGY: Pharmacokinetics, Special Populations, Clinical Studies .
IRBESARTAN ADVERSE REACTIONS
Hypertension
Body as a Whole
Cardiovascular:
Dermatologic
Endocrine/Metabolic/Electrolyte Imbalances
Gastrointestinal:
Musculoskeletal/Connective Tissue
Nervous System:
Renal/Genitourinary:
Respiratory:
Special Senses:
Nephropathy in Type 2 Diabetic Patients
Post-Marketing Experience
Laboratory Test Findings
Hypertension
Creatinine, Blood Urea Nitrogen:PRECAUTIONS: Impaired Renal Function.
Hematologic:3
Nephropathy in Type 2 Diabetic Patients
Hyperkalemia:
OVERDOSAGE
Physicians’ DeskReference
2
DOSAGE & ADMINISTRATION
Hypertension
CLINICAL PHARMACOLOGY: Clinical Studies
Nephropathy in Type 2 Diabetic Patients
CLINICAL PHARMACOLOGY: Clinical Studies
Volume- and Salt-Depleted Patients
WARNINGS: Hypotension in Volume- or Salt-Depleted Patients
HOW SUPPLIED
Storage
Store at 20° to 25° C (68° to 77° F) [see USP Controlled Room Temperature]


SPL PATIENT PACKAGE INSERT
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Container Label of 75 mg 30's count:

Container Label of 150 mg 30's count:

Container Label of 300 mg 30's count:

IrbesartanIrbesartan TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
IrbesartanIrbesartan TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
IrbesartanIrbesartan TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!