Ibuprofen
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- IBUPROFEN DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- IBUPROFEN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- IBUPROFEN ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
Cardiovascular Risk-
● NSAIDs may cause an increased risk of serious cardiovascularthrombotic events, myocardial infarction, and stroke,which can be fatal. This risk may increase with duration ofuse. Patients with cardiovascular disease or risk factors forcardiovascular disease may be at greater risk (SeeWARNINGS).
-
● IBU tablets are contraindicated for treatment of peri-operativepain in the setting of coronary artery bypass graft (CABG)surgery (SeeWARNINGS).
-
● NSAIDS cause an increased risk of serious gastrointestinaladverse events including bleeding, ulceration, and perforationof the stomach or intestines, which can be fatal. These eventscan occur at any time during use and without warning symptoms.Elderly patients are at greater risk for serious gastrointestinalevents. (SeeWARNINGS).
IBUPROFEN DESCRIPTION
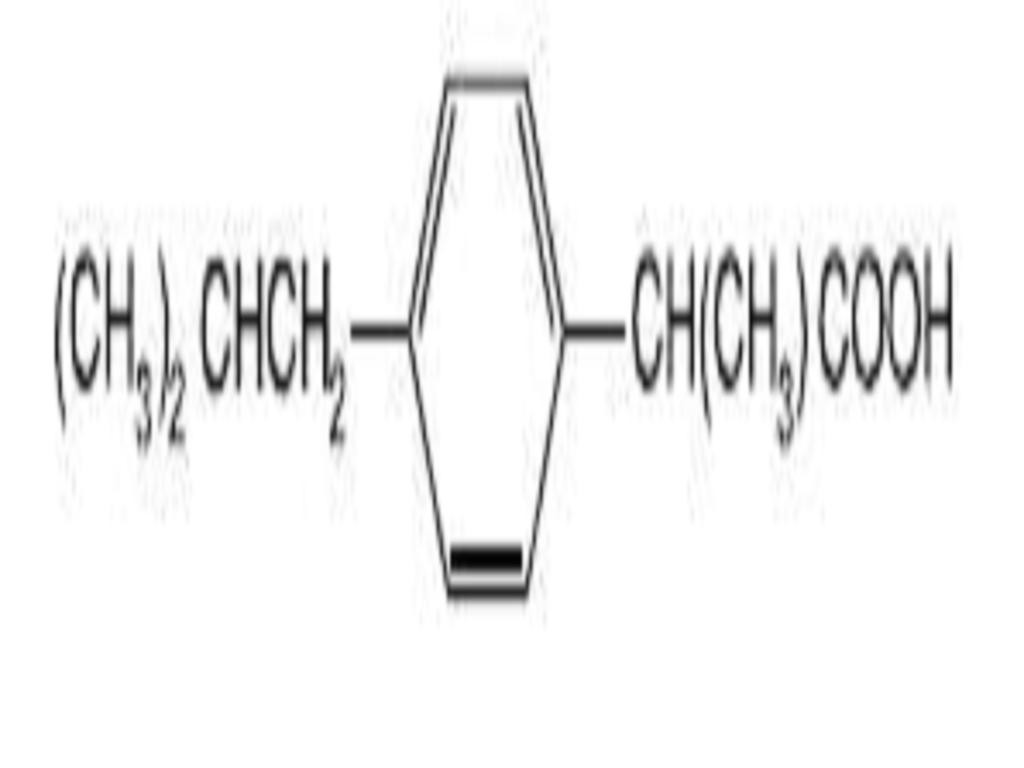
CLINICAL PHARMACOLOGY
ADVERSE REACTIONS
ADVERSE REACTIONS
INDICATIONS & USAGE
WARNINGSIBUPROFEN CONTRAINDICATIONS
WARNINGS, Anaphylactoid Reactions,PRECAUTIONS, Preexisting Asthma
WARNINGS
WARNINGS
CARDIOVASCULAR EFFECTSCardiovascular Thrombotic Events
WARNINGS)
CONTRAINDICATIONS
Hypertension
Congestive Heart Failure and Edema
Gastrointestinal Effects - Risk of Ulceration, Bleeding, and Perforation
Renal Effects
Advanced Renal Disease
Anaphylactoid Reactions
CONTRAINDICATIONSPRECAUTIONS, Preexisting Asthma)
Skin Reactions
Pregnancy
PRECAUTIONS
GeneralHepatic effects
Hematological effects
Preexisting asthma
Ophthalmological effects.
Aseptic Meningitis
INFORMATION FOR PATIENTS
WARNINGS,Cardiovascular Effects
WARNINGS,Gastrointestinal Effects-Risk of UlcerationBleeding and Perforation)
WARNINGS)
LABORATORY TESTS
DRUG INTERACTIONS
ACE-inhibitors:Aspirin
Diuretics
Lithium
Methotrexate
Warfarin-type anticoagulants
H-2 Antagonists
PREGNANCY
Teratogenic effects: Pregnancy Category CNonteratogenic effects
LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
IBUPROFEN ADVERSE REACTIONS
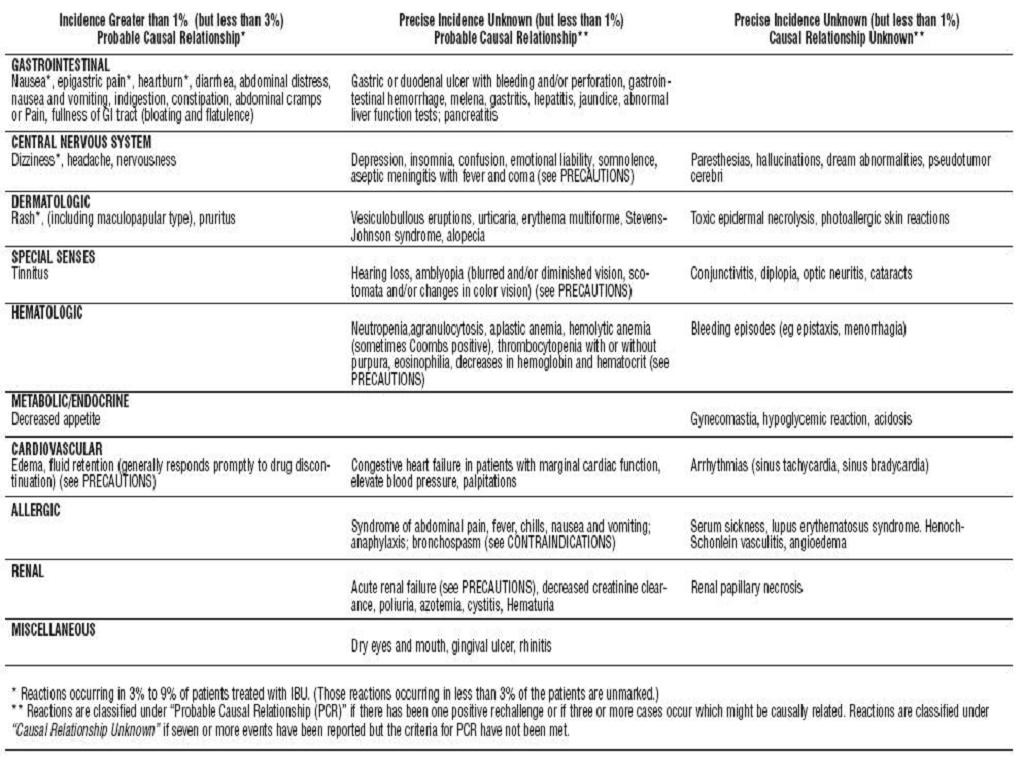
OVERDOSAGE
DOSAGE & ADMINISTRATION
Rheumatoid arthritis and osteoarthritis, including flare-ups ofchronic disease:
Mild to moderate pain:
Dysmenorrhea:
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

IbuprofenIbuprofen TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!