HYOSCYAMINE SULFATE
HYOSCYAMINE SULFATE ORAL DROPS
FULL PRESCRIBING INFORMATION: CONTENTS*
- Hyoscyamine Sulfate Oral Drops
- CLINICAL PHARMACOLOGY:
- HYOSCYAMINE SULFATE INDICATIONS AND USAGE:
- HYOSCYAMINE SULFATE CONTRAINDICATIONS:
- WARNINGS:
- PRECAUTIONS:
- HYOSCYAMINE SULFATE ADVERSE REACTIONS:
- OVERDOSAGE:
- HYOSCYAMINE SULFATE DOSAGE AND ADMINISTRATION:
- HOW SUPPLIED:
- PRODUCT PACKAGING:
FULL PRESCRIBING INFORMATION
Hyoscyamine Sulfate Oral Drops
(Hyoscyamine Sulfate Oral Solution)DESCRIPTION:
172332242
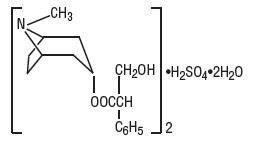
Hyoscyamine Sulfate Oral Drops
CLINICAL PHARMACOLOGY:
INDICATIONS AND USAGE:
CONTRAINDICATIONS:
WARNINGS:
PRECAUTIONS:
General:Information for Patients:
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Pregnancy - Pregnancy Category C:
Nursing Mothers:
Geriatric Use:
ADVERSE REACTIONS:
OVERDOSAGE:
50
DOSAGE AND ADMINISTRATION:
Adults and pediatric patients 12 years of age and older:
Pediatric patients 2 to under 12 years of age:
Pediatric patients under 2 years of age:
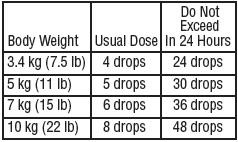
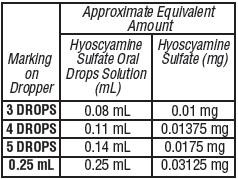
HOW SUPPLIED:
Rx Only
PRODUCT PACKAGING:
HYOSCYAMINE SULFATE
ORAL DROPS
Rx Only
CYPRESS
PHARMACEUTICAL, INC.
DO NOT USE IF TAMPER-EVIDENT SEAL IS BROKEN OR MISSING.
DOSAGE:TAKE BY MOUTH ONLY.
See package insert for full prescribing information.
CAUTION:
WARNING: KEEP OUT OF REACH OF CHILDREN. IN CASE OF ACCIDENTAL
OVERDOSE, SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON
CONTROL CENTER IMMEDIATELY.



HYOSCYAMINE SULFATEHyoscyamine Sulfate SOLUTION/ DROPS
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!