Hylartin V
Pharmacia & Upjohn Company
Pfizer Inc.
HYLARTIN V sodium hyaluronate injection
FULL PRESCRIBING INFORMATION: CONTENTS*
- CAUTION
- HYLARTIN V DESCRIPTION
- CHEMISTRY
- PHARMACOLOGY
- TOXICOLOGY
- CLINICAL STUDIES
- INDICATIONS
- HYLARTIN V CONTRAINDICATIONS
- WARNING
- PRECAUTIONS
- HYLARTIN V ADVERSE REACTIONS
- HYLARTIN V DOSAGE AND ADMINISTRATION
- STORAGE CONDITIONS
- HOW SUPPLIED
- REFERENCE
- INSTRUCTIONS
- PRINCIPAL DISPLAY PANEL - 2 mL Syringe Label
FULL PRESCRIBING INFORMATION
10 mg/mL
Product Information
CAUTION
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
HYLARTIN V DESCRIPTION
HYLARTIN® V is a sterile pyrogen-free solution of a highly purified, specific fraction of the sodium salt of hyaluronic acid extracted from rooster combs. HYLARTIN® V is supplied in disposable glass syringes, each of which contains 20 mg (10 mg/mL) of sodium hyaluronate in 2.0 mL physiological sodium chloride-phosphate buffer with a pH of 7.0–7.5.
CHEMISTRY
Sodium hyaluronate is a high molecular weight polymer made up of repeating disaccharide units of N-acetylglucosamine and sodium glucuronate linked by beta 1-3 and beta 1-4 glycosidic bonds. HYLARTIN® V contains only traces of protein.
PHARMACOLOGY
Sodium hyaluronate is a natural, physiological substance which occurs extracellularly in connective tissue in both animals and man and is chemically identical in different species. High concentrations (>0.2 mg/mL) of hyaluronate are found in the synovial fluid, the vitreous of the eye and the umbilical cord.
Sodium hyaluronate is a normal component of connective tissue matrix and it is injected therapeutically only in compartments where it constitutes a normal component, specifically the joint cavity.
TOXICOLOGY
Acute, sub-acute and chronic toxicity studies in mice, rats, rabbits, dogs, monkeys and horses have not demonstrated any significant adverse reactions or sensitization.
In an acute toxicity study in horses, HYLARTIN® V was injected intra-articularly at dosages corresponding to five times the recommended dose per animal (200 mg total). In a sub-acute study, horses were injected intra-articularly with the recommended dose per joint (20 mg) at weekly intervals for nine weeks. The results of both investigations showed that hematological and blood chemistry values remained within normal ranges. In mice, the intravenous LD100 was found to be of the order of 50 mg/kg body weight.
There is always a potential immunological risk with repeated parenteral administration of biological material. However, as shown by Richter (1974), sodium hyaluronate, of both human and avian origin, did not produce any antibodies after repeated immunization, nor did intense stimulation of the immunization process by coupling of protein to the hyaluronate and simultaneous administration of Freund's adjuvant give rise to antibodies.
CLINICAL STUDIES
Clinical field trials with thoroughbred and standardbred race horses were undertaken at four separate clinics. A total of 252 joints were injected with HYLARTIN® V in these investigations. In one study, only horses which were conventional treatment failures were included and the overall improvement rate following HYLARTIN® V treatment approached 90 percent. In the other studies, the improvement rate surpassed this figure.
In another case, electrogoniometry was used to objectively show that HYLARTIN® V can improve the function of arthritic carpal and fetlock joints. HYLARTIN® V brought return to symmetry with respect to timing and duration of various angular motions of the joints. In cases where HYLARTIN® V was not able to achieve contralateral symmetry of the joint motion pattern, blocking of the joint with anesthetic also had no effect, indicating that most probably mechanical damage was responsible for the joint dysfunction.
INDICATIONS
HYLARTIN® V, is indicated in the treatment of joint dysfunction in horses due to non-infectious synovitis associated with equine osteoarthritis.
HYLARTIN V CONTRAINDICATIONS
None known.
WARNING
Do not use in horses intended for human consumption.
HYLARTIN® V must not be administered intravascularly.
PRECAUTIONS
Used or partially used syringes should be crushed and disposed of in an approved landfill.
Do not use if numerous small air bubbles are present throughout the solution.
HYLARTIN V ADVERSE REACTIONS
The side effects observed in clinical trials were heat (15%), transient edema (12%), and pain (9%) around the treated joint. These side effects have been observed after intra-articular injection. Most of these reactions were of mild nature and in no case did they require the discontinuance of treatment. These reactions subsided in 24 to 48 hours.
For a copy of the Material Safety Data Sheet (MSDS) call 1-800-733-5500. To report adverse reactions call Pfizer Animal Health at 1-800-366-5288
HYLARTIN V DOSAGE AND ADMINISTRATION
2 mL (20 mg) of HYLARTIN® V given to horses intra-articularly in small and medium size joints (carpal, fetlock). In the treatment of larger joints (hock), the dosage is 4 mL (40 mg). The treatment may be repeated at weekly intervals for a total of three treatments.
HYLARTIN® V should be injected in horses intra-articularly under strict aseptic conditions. Effusion should be removed prior to injection. When performing the injections, care should be taken not to scratch the cartilage surface, as this may result in diffuse swelling lasting for 24 to 48 hours. This transient swelling, however, will have no effect on the ultimate clinical result. For best results, the horse should be given two days stall rest before gradually resuming normal activity.
STORAGE CONDITIONS
Store at 2° to 8°C. The expiration date is stated on the package.
Protect from freezing. Protect from light.
HOW SUPPLIED
HYLARTIN® V, is supplied sterile in disposable glass syringes, each containing 20 mg (10 mg/mL) of sodium hyaluronate in 2.0 mL physiological sodium chloride-phosphate buffer. Each mL contains: Sodium hyaluronate 10.0 mg, sodium chloride 8.5 mg, disodium hydrogen phosphate dihydrate 0.28 mg, sodium dihydrogen phosphate hydrate 0.04 mg, water for injection USP q.s.
REFERENCE
Richter, W., "Non-immunogenicity of purified hyaluronic acid preparations tested by passive cutaneous anaphylaxis", Int. Arch. Allergy 47:211–217, 1974.
NADA 112-048, Approved by FDA
Made in Sweden by:
Advanced Medical Optics Uppsala AB
Box 6406, SE-751 36 Uppsala, Sweden
For:
Pharmacia & Upjohn Company
Division of Pfizer Inc
New York, NY 10017
Revised: June 2011
52-0199-00
INSTRUCTIONS
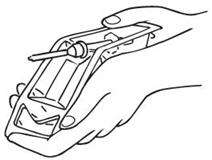
OPENING TECHNIQUE
|
Tear off the paper covering.
Bend the plastic backwards at the central indentation so as to fully expose the white plastic cap. Dislodge syringe. |
|
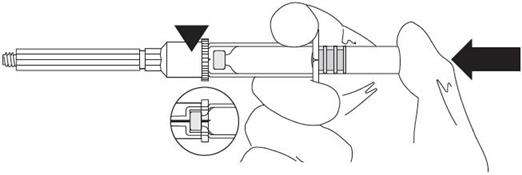
ASSEMBLY
| Press the vial completely into the holder until the needle perforates the membrane. |
|
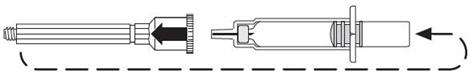
| Remove the plastic cap. |
|
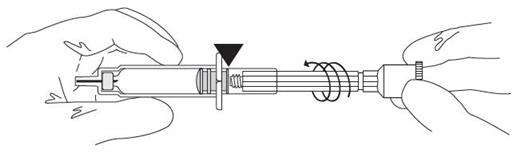
| Screw the plastic cap into the blue plunger. |
|
| Connect the cannula and check for proper function. |
|
Store at 2° to 8°C.
For single use only.
820 367 002
52-0199-00
PRINCIPAL DISPLAY PANEL - 2 mL Syringe Label
2 mL
HYLARTIN
®
V
sodium hyaluronate
injection
10 mg/mL
| CAUTION Federal law restricts this drug to use by or on the order of a licensed veterinarian. |
Sterile
Contents: one 2 mL syringe
Each mL contains: Sodium
hyaluronate 10.0 mg, sodium
chloride 8.5 mg, disodium
hydrogen phosphate
dihydrate 0.28 mg, sodium
dihydrogen phosphate
hydrate 0.04 mg, water for
injection USP q.s.
Do not use in horses intended for
human consumption.
Dosage: 2 mL carpal-fetlock/
4 mL, hock.
See package insert.
Do not use if numerous small
air bubbles are present
throughout the solution.
For intra-articular injection in
horses only.
Store at 2° to 8°C.
Protect from freezing.
Protect from light.
NADA 112-048, Approved by FDA
Made in Sweden by:
Advanced Medical Optics Uppsala AB
Box 6406,
SE-751 36 Uppsala, Sweden
For: Pharmacia & Upjohn Co.
Division of Pfizer Inc
New York, NY 10017
51-0048-00
820 365 001 1432000
Lot No:
Expiration:

Hylartin Vhyaluronate sodium INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||