HYDROCODONE BITARTRATE AND ACETAMINOPHEN
McKesson Packaging Services a business unit of McKesson Corporation
McKesson Packaging Services a business unit of McKesson Corporation
HYDROCODONE BITARTRATE AND ACETAMINOPHEN TABLETS USP CIII
FULL PRESCRIBING INFORMATION: CONTENTS*
- HYDROCODONE BITARTRATE AND ACETAMINOPHEN DESCRIPTION
- CLINICAL PHARMACOLOGY
- HYDROCODONE BITARTRATE AND ACETAMINOPHEN INDICATIONS AND USAGE
- HYDROCODONE BITARTRATE AND ACETAMINOPHEN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- HYDROCODONE BITARTRATE AND ACETAMINOPHEN ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- HYDROCODONE BITARTRATE AND ACETAMINOPHEN DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- REPRESENTATIVE PACKAGING
FULL PRESCRIBING INFORMATION
| 5 mg/325 mg | 5 mg/500 mg | |||
| 7.5 mg/325 mg | 7.5 mg/500 mg | 7.5 mg/650 mg | 7.5 mg/750 mg | |
| 10 mg/325 mg | 10 mg/500 mg | 10 mg/650 mg | 10 mg/660 mg | 10 mg/750 mg |
Rx only
HYDROCODONE BITARTRATE AND ACETAMINOPHEN DESCRIPTION
Hydrocodone Bitartrate and Acetaminophen Tablets are supplied in tablet form for oral administration.
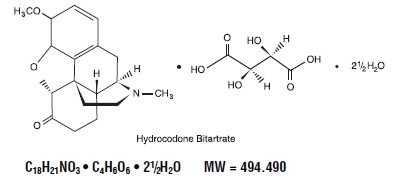
Hydrocodone bitartrate is an opioid analgesic and antitussive and occurs as fine, white crystals or as a crystalline powder. It is affected by light. The chemical name is: 4,5α-epoxy-3-methoxy-17-methylmorphinan-6-one tartrate (1:1) hydrate (2:5). It has the following structural formula:
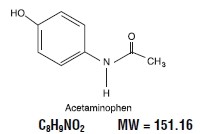
Acetaminophen, 4'-hydroxyacetanilide, a slightly bitter, white, odorless, crystalline powder, is a non-opiate, non-salicylate analgesic and antipyretic. It has the following structural formula:
Each Hydrocodone Bitartrate and Acetaminophen Tablet USP contains:
| Tablet | Hydrocodone Bitartrate USP | Acetaminophen USP |
| 5 mg/325 mg | 5 mg | 325 mg |
| 5 mg/500 mg | 5 mg | 500 mg |
| 7.5 mg/325 mg | 7.5 mg | 325 mg |
| 7.5 mg/500 mg | 7.5 mg | 500 mg |
| 7.5 mg/650 mg | 7.5 mg | 650 mg |
| 7.5 mg/750 mg | 7.5 mg | 750 mg |
| 10 mg/325 mg | 10 mg | 325 mg |
| 10 mg/500 mg | 10 mg | 500 mg |
| 10 mg/650 mg | 10 mg | 650 mg |
| 10 mg/660 mg | 10 mg | 660 mg |
| 10 mg/750 mg | 10 mg | 750 mg |
In addition, each tablet contains the following inactive ingredients: crospovidone, magnesium stearate, microcrystalline cellulose, povidone, pregelatinized starch, silicon dioxide, and stearic acid.
The 10 mg/650 mg tablet also contains FD & C Blue No. 1 Aluminum Lake 12%.
Meets USP Dissolution Test 1.
CLINICAL PHARMACOLOGY
Hydrocodone is a semisynthetic narcotic analgesic and antitussive with multiple actions qualitatively similar to those of codeine. Most of these involve the central nervous system and smooth muscle. The precise mechanism of action of hydrocodone and other opiates is not known, although it is believed to relate to the existence of opiate receptors in the central nervous system. In addition to analgesia, narcotics may produce drowsiness, changes in mood and mental clouding.
The analgesic action of acetaminophen involves peripheral influences, but the specific mechanism is as yet undetermined. Antipyretic activity is mediated through hypothalamic heat regulating centers. Acetaminophen inhibits prostaglandin synthetase. Therapeutic doses of acetaminophen have negligible effects on the cardiovascular or respiratory systems; however, toxic doses may cause circulatory failure and rapid, shallow breathing.
Pharmacokinetics – The behavior of the individual components is described below.
Hydrocodone – Following a 10 mg oral dose of hydrocodone administered to five adult male subjects, the mean peak concentration was 23.6 ± 5.2 ng/mL. Maximum serum levels were achieved at 1.3 ± 0.3 hours and the half-life was determined to be 3.8 ± 0.3 hours. Hydrocodone exhibits a complex pattern of metabolism including O-demethylation, N-demethylation and 6-keto reduction to the corresponding 6-α- and 6-β-hydroxymetabolites (see OVERDOSAGE for toxicity information).
Acetaminophen – Acetaminophen is rapidly absorbed from the gastrointestinal tract and is distributed throughout most body tissues. The plasma half-life is 1.25 to 3 hours, but may be increased by liver damage and following overdosage. Elimination of acetaminophen is principally by liver metabolism (conjugation) and subsequent renal excretion of metabolites. Approximately 85% of an oral dose appears in the urine within 24 hours of administration, most as the glucuronide conjugate, with small amounts of other conjugates and unchanged drug (see OVERDOSAGE for toxicity information).
HYDROCODONE BITARTRATE AND ACETAMINOPHEN INDICATIONS AND USAGE
Hydrocodone bitartrate and acetaminophen tablets are indicated for the relief of moderate to moderately severe pain.
HYDROCODONE BITARTRATE AND ACETAMINOPHEN CONTRAINDICATIONS
This product should not be administered to patients who have previously exhibited hypersensitivity to hydrocodone or acetaminophen.
Patients known to be hypersensitive to other opioids may exhibit cross-sensitivity to hydrocodone.
WARNINGS
Respiratory Depression – At high doses or in sensitive patients, hydrocodone may produce dose-related respiratory depression by acting directly on the brain stem respiratory center. Hydrocodone also affects the center that controls respiratory rhythm, and may produce irregular and periodic breathing.
Head Injury and Increased Intracranial Pressure – The respiratory depressant effects of narcotics and their capacity to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions or a pre-existing increase in intracranial pressure. Furthermore, narcotics produce adverse reactions which may obscure the clinical course of patients with head injuries.
Acute Abdominal Conditions – The administration of narcotics may obscure the diagnosis or clinical course of patients with acute abdominal conditions.
PRECAUTIONS
General
Special Risk Patients – As with any narcotic analgesic agent, hydrocodone bitartrate and acetaminophen tablets should be used with caution in elderly or debilitated patients, and those with severe impairment of hepatic or renal function, hypothyroidism, Addison's disease, prostatic hypertrophy or urethral stricture. The usual precautions should be observed and the possibility of respiratory depression should be kept in mind.
Cough Reflex – Hydrocodone suppresses the cough reflex; as with all narcotics, caution should be exercised when hydrocodone bitartrate and acetaminophen tablets are used post-operatively and in patients with pulmonary disease.
Information for Patients – Hydrocodone, like all narcotics, may impair mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery; patients should be cautioned accordingly.
Alcohol and other CNS depressants may produce an additive CNS depression, when taken with this combination product, and should be avoided.
Hydrocodone may be habit-forming. Patients should take the drug only for as long as it is prescribed, in the amounts prescribed, and no more frequently than prescribed.
Laboratory Tests – In patients with severe hepatic or renal disease, effects of therapy should be monitored with serial liver and/or renal function tests.
Drug Interactions – Patients receiving narcotics, antihistamines, antipsychotics, antianxiety agents, or other CNS depressants (including alcohol) concomitantly with hydrocodone bitartrate and acetaminophen tablets may exhibit an additive CNS depression. When combined therapy is contemplated, the dose of one or both agents should be reduced.
The use of MAO inhibitors or tricyclic antidepressants with hydrocodone preparations may increase the effect of either the antidepressant or hydrocodone.
Drug/Laboratory Test Interactions – Acetaminophen may produce false-positive test results for urinary 5-hydroxyindoleacetic acid.
Carcinogenesis, Mutagenesis, Impairment of Fertility – No adequate studies have been conducted in animals to determine whether hydrocodone or acetaminophen have a potential for carcinogenesis, mutagenesis, or impairment of fertility.
Pregnancy
Teratogenic Effects. Pregnancy Category C – There are no adequate and well-controlled studies in pregnant women. Hydrocodone bitartrate and acetaminophen tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects – Babies born to mothers who have been taking opioids regularly prior to delivery will be physically dependent. The withdrawal signs include irritability and excessive crying, tremors, hyperactive reflexes, increased respiratory rate, increased stools, sneezing, yawning, vomiting, and fever. The intensity of the syndrome does not always correlate with the duration of maternal opioid use or dose. There is no consensus on the best method of managing withdrawal.
Labor and Delivery – As with all narcotics, administration of this product to the mother shortly before delivery may result in some degree of respiratory depression in the newborn, especially if higher doses are used.
Nursing Mothers – Acetaminophen is excreted in breast milk in small amounts, but the significance of its effects on nursing infants is not known. It is not known whether hydrocodone is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from hydrocodone and acetaminophen, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use – Safety and effectiveness in pediatric patients have not been established.
Geriatric Use – Clinical studies of hydrocodone bitartrate and acetaminophen tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Hydrocodone and the major metabolites of acetaminophen are known to be substantially excreted by the kidney. Thus the risk of toxic reactions may be greater in patients with impaired renal function due to the accumulation of the parent compound and/or metabolites in the plasma. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Hydrocodone may cause confusion and over-sedation in the elderly; elderly patients generally should be started on low doses of hydrocodone bitartrate and acetaminophen tablets and observed closely.
HYDROCODONE BITARTRATE AND ACETAMINOPHEN ADVERSE REACTIONS
The most frequently reported adverse reactions are light-headedness, dizziness, sedation, nausea and vomiting. These effects seem to be more prominent in ambulatory than in non-ambulatory patients, and some of these adverse reactions may be alleviated if the patient lies down.
Other adverse reactions include:
Central Nervous System – Drowsiness, mental clouding, lethargy, impairment of mental and physical performance, anxiety, fear, dysphoria, psychic dependence, mood changes.
Gastrointestinal System – Prolonged administration of hydrocodone bitartrate and acetaminophen tablets may produce constipation.
Genitourinary System – Ureteral spasm, spasm of vesical sphincters and urinary retention have been reported with opiates.
Respiratory Depression – Hydrocodone bitartrate may produce dose-related respiratory depression by acting directly on the brain stem respiratory centers (see OVERDOSAGE ).
Special Senses – Cases of hearing impairment or permanent loss have been reported predominantly in patients with chronic overdose.
Dermatological – Skin rash, pruritus.
The following adverse drug events may be borne in mind as potential effects of acetaminophen: allergic reactions, rash, thrombocytopenia, agranulocytosis.
Potential effects of high dosage are listed in the OVERDOSAGE section.
DRUG ABUSE AND DEPENDENCE
Controlled Substance – Hydrocodone bitartrate and acetaminophen tablets are classified as a Schedule III controlled substance.
Abuse and Dependence – Psychic dependence, physical dependence, and tolerance may develop upon repeated administration of narcotics; therefore, this product should be prescribed and administered with caution. However, psychic dependence is unlikely to develop when hydrocodone bitartrate and acetaminophen tablets are used for a short time for the treatment of pain.
Physical dependence, the condition in which continued administration of the drug is required to prevent the appearance of a withdrawal syndrome, assumes clinically significant proportions only after several weeks of continued narcotic use, although some mild degree of physical dependence may develop after a few days of narcotic therapy. Tolerance, in which increasingly large doses are required in order to produce the same degree of analgesia, is manifested initially by a shortened duration of analgesic effect, and subsequently by decreases in the intensity of analgesia. The rate of development of tolerance varies among patients.
OVERDOSAGE
Following an acute overdosage, toxicity may result from hydrocodone or acetaminophen.
Signs and Symptoms
Hydrocodone – Serious overdose with hydrocodone is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, and sometimes bradycardia and hypotension. In severe overdosage, apnea, circulatory collapse, cardiac arrest and death may occur.
Acetaminophen – In acetaminophen overdosage: dose-dependent, potentially fatal hepatic necrosis is the most serious adverse effect. Renal tubular necrosis, hypoglycemic coma, and thrombocytopenia may also occur.
Early symptoms following a potentially hepatotoxic overdose may include: nausea, vomiting, diaphoresis and general malaise. Clinical and laboratory evidence of hepatic toxicity may not be apparent until 48 to 72 hours post-ingestion.
In adults, hepatic toxicity has rarely been reported with acute overdoses of less than 10 grams and fatalities with less than 15 grams.
Treatment – A single or multiple overdose with hydrocodone and acetaminophen is a potentially lethal polydrug overdose, and consultation with a regional poison control center is recommended.
Immediate treatment includes support of cardiorespiratory function and measures to reduce drug absorption. Vomiting should be induced mechanically, or with syrup of ipecac, if the patient is alert (adequate pharyngeal and laryngeal reflexes). Oral activated charcoal (1 g/kg) should follow gastric emptying. The first dose should be accompanied by an appropriate cathartic. If repeated doses are used, the cathartic might be included with alternate doses as required. Hypotension is usually hypovolemic and should respond to fluids. Vasopressors and other supportive measures should be employed as indicated. A cuffed endo-tracheal tube should be inserted before gastric lavage of the unconscious patient and, when necessary, to provide assisted respiration.
Meticulous attention should be given to maintaining adequate pulmonary ventilation. In severe cases of intoxication, peritoneal dialysis, or preferably hemodialysis may be considered. If hypoprothrombinemia occurs due to acetaminophen overdose, vitamin K should be administered intravenously.
Naloxone, a narcotic antagonist, can reverse respiratory depression and coma associated with opioid overdose. Naloxone hydrochloride 0.4 mg to 2 mg is given parenterally. Since the duration of action of hydrocodone may exceed that of naloxone, the patient should be kept under continuous surveillance and repeated doses of the antagonist should be administered as needed to maintain adequate respiration. A narcotic antagonist should not be administered in the absence of clinically significant respiratory or cardiovascular depression.
If the dose of acetaminophen may have exceeded 140 mg/kg, acetylcysteine should be administered as early as possible. Serum acetaminophen levels should be obtained, since levels four or more hours following ingestion help predict acetaminophen toxicity. Do not await acetaminophen assay results before initiating treatment. Hepatic enzymes should be obtained initially, and repeated at 24-hour intervals.
Methemoglobinemia over 30% should be treated with methylene blue by slow intravenous administration.
The toxic dose of acetaminophen for adults is 10 grams.
HYDROCODONE BITARTRATE AND ACETAMINOPHEN DOSAGE AND ADMINISTRATION
Dosage should be adjusted according to the severity of pain and response of the patient. However, it should be kept in mind that tolerance to hydrocodone can develop with continued use and that the incidence of untoward effects is dose related.
The usual adult dosage for Hydrocodone Bitartrate and Acetaminophen Tablets USP is:
|
Product Strength |
Usual Adult Dosage as needed for pain |
The total 24-hour dosage should not exceed |
| 5 mg/325 mg | One to two tablets every four to six hours | 12 tablets |
| 5 mg/500 mg | One to two tablets every four to six hours | 8 tablets |
| 7.5 mg/325 mg | One tablet every four to six hours | 8 tablets |
| 7.5 mg/500 mg | One tablet every four to six hours | 6 tablets |
| 7.5 mg/650 mg | One tablet every four to six hours | 6 tablets |
| 7.5 mg/750 mg | One tablet every four to six hours | 5 tablets |
| 10 mg/325 mg | One tablet every four to six hours | 6 tablets |
| 10 mg/500 mg | One tablet every four to six hours | 6 tablets |
| 10 mg/650 mg | One tablet every four to six hours | 6 tablets |
| 10 mg/660 mg | One tablet every four to six hours | 6 tablets |
| 10 mg/750 mg | One tablet every four to six hours | 5 tablets |
HOW SUPPLIED
Hydrocodone Bitartrate and Acetaminophen Tablets USP are supplied as follows:
| Strength | How Supplied | Each tablet contains: | Description of tablet | |
| Hydrocodone Bitartrate | Acetaminophen | |||
| 5 mg/500 mg | Bottles of 60 NDC 63739-801-42 Bottles of 90 NDC 63739-801-43 |
5 mg | 500 mg | It is available as a capsule-shaped white tablet debossed with M357 on one side and bisected on the other side. |
Dispense in a tight, light-resistant container (as defined in USP) with a child-resistant closure.
Storage – Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from light.
A Schedule III Narcotic.
Mallinckrodt Inc.
Hazelwood, MO 63042 USA
Repackaged by RxPak
Division of McKesson Corp.
Memphis, TN 38141
Distributed By:
McKesson Packaging Services
a business unit of McKesson Corporation
7101 Weddington Rd., Concord, NC 28027
IS-4010986
Rev. 10/2009
REPRESENTATIVE PACKAGING
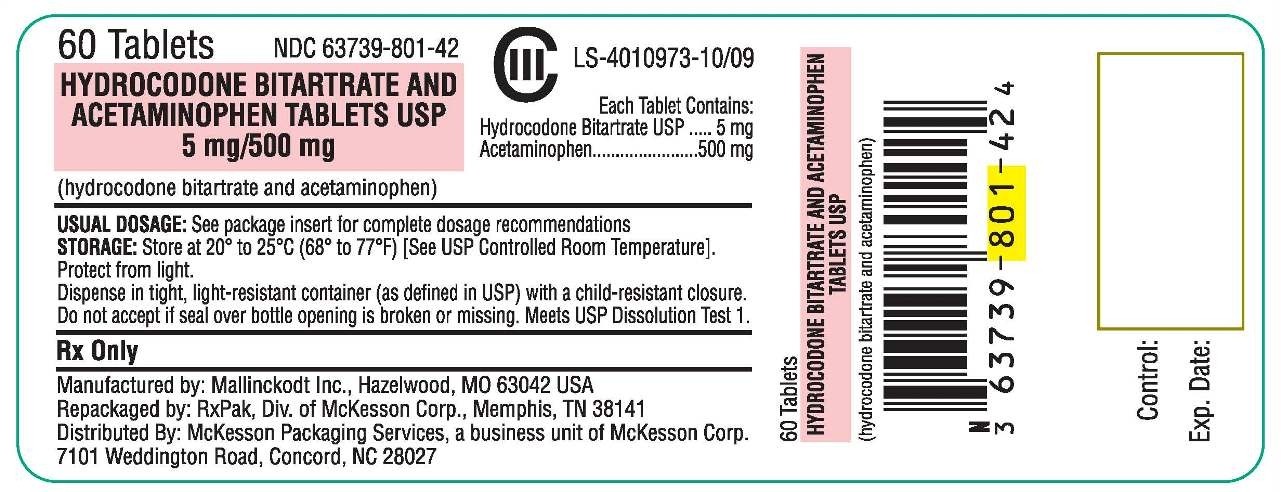
HYDROCODONE BITARTRATE AND ACETAMINOPHENHYDROCODONE BITARTRATE AND ACETAMINOPHEN TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||