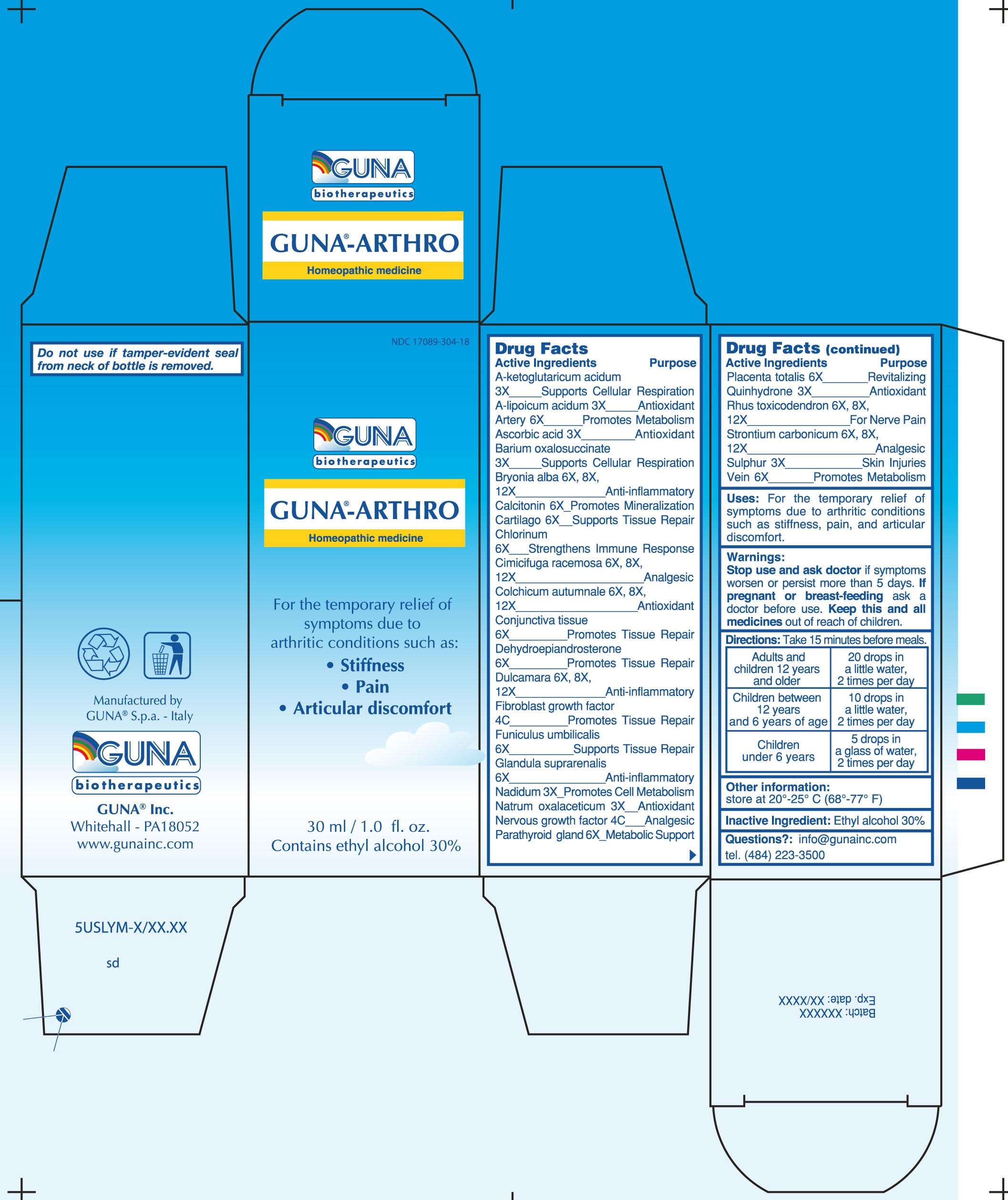
GUNA-ARTHRO
DRUG FACTS
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENTS/PURPOSE
A-KETOGLUTARICUM ACIDUM 3X SUPPORTS CELLULAR RESPIRATION
A-LIPOICUM ACIDUM 3X ANTIOXIDANT
ARTERY 6X PROMOTES METABOLISM
ASCORBIC ACID 3X ANTIOXIDANT
BARIUM OXALOSUCCINATE 3X SUPPORTS CELLULAR RESPIRATION
BRYONIA ALBA 6X, 8X, 12X ANTI-INFLAMMATORY
CALCITONIN 6X PROMOTES MINERALIZATION
CARTILAGO 6X SUPPORTS TISSUE REPAIR
CHLORINUM 6X STRENGTHENS IMMUNE RESPONSE
CIMICIFUGA RACEMOSA 6X, 8X, 12X ANALGESIC
COLCHICUM AUTUMNALE 6X, 8X, 12X ANTIOXIDANT
CONJUNCTIVA TISSUE 6X PROMOTES TISSUE REPAIR
DEHYDROEPIANDROSTERONE 6X PROMOTES TISSUE REPAIR
DULCAMARA 6X, 8X, 12X ANTI-INFLAMMATORY
FIBROBLAST GROWTH FACTOR 4C PROMOTES TISSUE REPAIR
FUNICULUS UMBILICALIS 6X SUPPORTS TISSUE REPAIR
GLANDULA SUPRARENALIS 6X ANTI-INFLAMMATORY
NADIDUM 3X PROMOTES CELL METABOLISM
NATRUM OXALACETICUM 3X ANTIOXIDANT
NERVOUS GROWTH FACTOR 4C ANALGESIC
PARATHYROID GLAND 6X METABOLIC SUPPORT
PLACENTA TOTALIS 6X REVITALIZING
QUINHYDRONE 3X ANTIOXIDANT
RHUS TOXICODENDRON 6X, 8X, 12X FOR NERVE PAIN
STRONTIUM CARBONICUM 6X, 8X, 12X ANALGESIC
SULPHUR 3X SKIN INJURIES
VEIN 6X PROMOTES METABOLISM
USES
For the temporary relief of symptoms due to arthritic conditions such as: stiffness, pain, and articular discomfort
WARNINGS
Stop use and ask doctor if symptoms worsen or persist more than 5 days
PREGNANCY
If pregnant or breast-feeding ask a doctor before use
WARNINGS
Keep this and all medicines out of reach of children
DIRECTIONS
Take 15 minutes before meals
Adults and children 12 years and older 20 drops in a little water 2 times per day
Children between 12 years and 6 years of age 10 drops in a little water 2 times per day
Children under 6 years 5 drops in a little water 2 times per day
QUESTIONS
Questions?: info@gunainc.com
Tel. (484) 223-3500
PRINCIPAL DISPLAY PANEL
GUNA-ARTHRO.ALPHA.-KETOGLUTARIC ACID - ALPHA LIPOIC ACID - ASCORBIC ACID - BARIUM OXALOSUCCINATE - BLACK COHOSH - BRYONIA ALBA ROOT - CALCITONIN HUMAN - CHLORINE - COLCHICUM AUTUMNALE BULB - NADIDE - SOLUTION/ DROPS
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||