GolfersSkin Sunscreen Zinc
GolfersSkin Sunscreen Zinc
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredients
Octinoxate 7.5%
Titanium Dioxide 2.35%
Zinc Oxide 4.2%
Purpose
Purpose
Sunscreen
Uses
Uses
Helps prevent sunburn
Higher SPF gives more sunburn protection
Provides high protection against sunburn
Regains SPF after 80 minutes of sweating.
Warnings
For external use only
UV exposure from the sun increases the risk of skin cancer, premature skin aging and other skin damage. It is important to decrease UV exposure by limiting time in the sun, wearing protective clothing and using a sunscreen.
When using this product
Keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor
if irritation occurs or rash develops and lasts.
Directions
apply generously 15mins before sun exposure and as needed
Children under 6 months of age: ask a doctor.
Reapply as needed or after towel drying, swimming or sweating.
Inactive Ingredients
Butylated Hydroxytoluene, Castor oil, Fragrance, Hydrogenated castor oil, Iron Oxides, Kaolin, Oleyl-alcohol, paraffin wax, petroleum jelly, sunflower oil, Talc
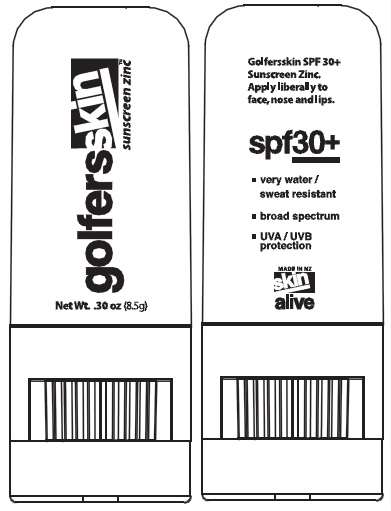
Golfersskin SPF 30+ Sunscreen Zinc gives great protection especially in the snow. Apply liberally to face, nose and lips.
To report a serious adverse event, contact 1-800-332-1088
Expires:
Lot Number:
ww.snowskin.co
DIST. BY Quiver Dist, Bend Oregon, USA 97701
Tested on Snowboarders - Not animals
AS/ANZ STANDARD 2604-1998
Net Wt. 0.30oz (8.5g)
SPF 30+
very water / sweat resistant
broad spectrum
UVA/UVB protection
Proven in extreme new zealand conditions
GolfersSkin sunscreen Zinc
hands free
Net Wt. 0.30oz (8.5g)
contains no nanoparticles-aminobenzoic acid (PABA) free

GolfersSkin Sunscreen ZincOCTINOXATE, TITANIUM DIOXIDE, ZINC OXIDE STICK
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||