Glipizide and Metformin Hydrochloride
CARACO PHARMACEUTICAL LABORATORIES, LTD
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- GLIPIZIDE AND METFORMIN HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- Clinical Studies
- GLIPIZIDE AND METFORMIN HYDROCHLORIDE INDICATIONS AND USAGE
- GLIPIZIDE AND METFORMIN HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- GLIPIZIDE AND METFORMIN HYDROCHLORIDE ADVERSE REACTIONS
- Hypoglycemia
- Gastrointestinal Reactions
- OVERDOSAGE
- GLIPIZIDE AND METFORMIN HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- STORAGE
- SUPPLEMENTAL PATIENT MATERIAL
FULL PRESCRIBING INFORMATION
BOXED WARNING
GLIPIZIDE AND METFORMIN HYDROCHLORIDE DESCRIPTION
Glipizide/Metformin hydrochloride Tablet contains two oral antihyperglycemic drugs used in the management of type 2 diabetes, glipizide and metformin hydrochloride.
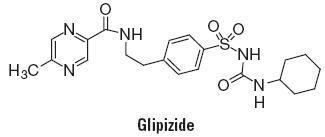
Glipizide is an oral antihyperglycemic drug of the sulfonylurea class. The chemical name for glipizide is 1-cyclohexyl-3-[[p-[2-(5-methylpyrazinecarboxamido)ethyl]phenyl]sulfonyl]urea. Glipizide is a whitish, odorless powder with a molecular formula of C21H27N5O4S, a molecular weight of 445.55 and a pKa of 5.9. It is insoluble in water and alcohols, but soluble in 0.1 N NaOH; it is freely soluble in dimethylformamide. The structural formula is represented below.
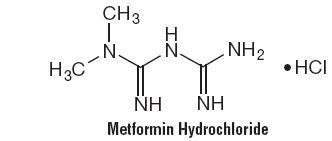
Metformin hydrochloride is an oral antihyperglycemic drug used in the management of type 2 diabetes. Metformin hydrochloride (N,N-dimethylimidodicarbonimidic diamide monohydrochloride) is not chemically or pharmacologically related to sulfonylureas, thiazolidinediones, or α-glucosidase inhibitors. It is a white to off-white crystalline compound with a molecular formula of C4H12ClN5 (monohydrochloride) and a molecular weight of 165.63. Metformin hydrochloride is freely soluble in water and is practically insoluble in acetone, ether, and chloroform. The pKa of metformin is 12.4. The pH of a 1% aqueous solution of metformin hydrochloride is 6.68. The structural formula is as shown:
Glipizide/metformin HCl Tablet is available for oral administration containing 2.5 mg glipizide with 250 mg metformin hydrochloride, 2.5 mg glipizide with 500 mg metformin hydrochloride, and 5 mg glipizide with 500 mg metformin hydrochloride. In addition, each tablet contains the following inactive ingredients: microcrystalline cellulose, povidone, croscarmellose sodium, polyethylene glycol and magnesium stearate. In addition, the coating for 2.5 mg/500 mg strength contains: polyvinyl alcohol, titanium dioxide, polyethylene glycol 3350 and talc. The coating for 2.5 mg/250 mg and 5 mg/500 mg strength contains: polyvinyl alcohol, titanium dioxide, polyethylene glycol 3350, talc, lecithin, iron oxide yellow and iron oxide red.
CLINICAL PHARMACOLOGY
Clinical Studies
GLIPIZIDE AND METFORMIN HYDROCHLORIDE INDICATIONS AND USAGE
Glipizide/metformin HCl tablet is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
GLIPIZIDE AND METFORMIN HYDROCHLORIDE CONTRAINDICATIONS
Glipizide/metformin HCl tablet is contraindicated in patients with:
- Renal disease or renal dysfunction (e.g., as suggested by serum creatinine levels ≥ 1.5 mg/dL [males], ≥ 1.4 mg/dL [females], or abnormal creatinine clearance) which may also result from conditions such as cardiovascular collapse (shock), acute myocardial infarction, and septicemia (see WARNINGS and PRECAUTIONS).
- Known hypersensitivity to glipizide or metformin hydrochloride.
- Acute or chronic metabolic acidosis, including diabetic ketoacidosis, with or without coma. Diabetic ketoacidosis should be treated with insulin.
Glipizide/metformin HCl Tablet should be temporarily discontinued in patients undergoing radiologic studies involving intravascular administration of iodinated contrast materials, because use of such products may result in acute alteration of renal function. (See also PRECAUTIONS.)
WARNINGS
Metformin Hydrochloride
SPECIAL WARNING ON INCREASED RISK OF CARDIOVASCULAR MORTALITY
The administration of oral hypoglycemic drugs has been reported to be associated with increased cardiovascular mortality as compared to treatment with diet alone or diet plus insulin. This warning is based on the study conducted by the University Group Diabetes Program (UGDP), a long-term prospective clinical trial designed to evaluate the effectiveness of glucose-lowering drugs in preventing or delaying vascular complications in patients with non-insulin-dependent diabetes. The study involved 823 patients who were randomly assigned to one of four treatment groups (Diabetes 19 (Suppl. 2):747-830, 1970).
UGDP reported that patients treated for 5 to 8 years with diet plus a fixed dose of tolbutamide (1.5 grams per day) had a rate of cardiovascular mortality approximately 2½ times that of patients treated with diet alone. A significant increase in total mortality was not observed, but the use of tolbutamide was discontinued based on the increase in cardiovascular mortality, thus limiting the opportunity for the study to show an increase in overall mortality. Despite controversy regarding the interpretation of these results, the findings of the UGDP study provide an adequate basis for this warning. The patient should be informed of the potential risks and benefits of glipizide and of alternative modes of therapy.
Although only one drug in the sulfonylurea class (tolbutamide) was included in this study, it is prudent from a safety standpoint to consider that this warning may also apply to other hypoglycemic drugs in this class, in view of their close similarities in mode of action and chemical structure.
PRECAUTIONS
General
Information for Patients
Laboratory Tests
Periodic fasting blood glucose and glycosylated hemoglobin (HbA1c) measurements should
be performed to monitor therapeutic response.
Initial and periodic monitoring of hematologic parameters (e.g., hemoglobin/hematocrit and red
blood cell indices) and renal function (serum creatinine) should be performed, at least on an
annual basis. While megaloblastic anemia has rarely been seen with metformin therapy, if this is
suspected, vitamin B12 deficiency should be excluded.
Drug Interactions
Carcinogenesis, Mutagenesis, Impairment of Fertility
No animal studies have been conducted with the combined products in glipizide/metformin HCl tablet. The following data are based on findings in studies performed with the individual products.
Pregnancy
Recent information strongly suggests that abnormal blood glucose levels during pregnancy
are associated with a higher incidence of congenital abnormalities. Most experts recommend that
insulin be used during pregnancy to maintain blood glucose as close to normal as possible. Because
animal reproduction studies are not always predictive of human response, glipizide/metformin HCl
tablet should not be used during pregnancy unless clearly needed. (See below.)
There are no adequate and well-controlled studies in pregnant women with glipizide/metformin HCl
tablet or its individual components. No animal studies have been conducted with the combined
products in glipizide/metformin HCl tablet. The following data are based on findings in studies
performed with the individual products.
Prolonged severe hypoglycemia (4 to 10 days) has been reported in neonates born to mothers who were receiving a sulfonylurea drug at the time of delivery. This has been reported more frequently with the use of agents with prolonged half-lives. It is not recommended that glipizide/metformin HCl tablet be used during pregnancy. However, if it is used, glipizide/metformin HCl tablet should be discontinued at least one month before the expected delivery date. (See Pregnancy: Teratogenic Effects: Pregnancy Category C.)
Nursing Mothers
Although it is not known whether glipizide is excreted in human milk, some sulfonylurea drugs are known to be excreted in human milk. Studies in lactating rats show that metformin is excreted into milk and reaches levels comparable to those in plasma. Similar studies have not been conducted in nursing mothers. Because the potential for hypoglycemia in nursing infants may exist, a decision should be made whether to discontinue nursing or to discontinue glipizide/metformin HCl tablet, taking into account the importance of the drug to the mother. If glipizide/metformin HCl tablet is discontinued, and if diet alone is inadequate for controlling blood glucose, insulin therapy should be considered.
Pediatric Use
Safety and effectiveness of glipizide/metformin HCl tablet in pediatric patients have not been established.
Geriatric Use
Of the 345 patients who received glipizide/metformin HCl tablet 2.5 mg/250 mg and 2.5
mg/500 mg in the initial therapy trial, 67 (19.4%) were aged 65 and older while 5 (1.4%) were aged
75 and older. Of the 87 patients who received glipizide/metformin HCl tablet in the second-line
therapy trial, 17 (19.5%) were aged 65 and older while one (1.1%) was at least aged 75. No overall
differences in effectiveness or safety were observed between these patients and younger patients in
either the initial therapy trial or the second-line therapy trial, and other reported clinical
experience has not identified differences in response between the elderly and younger patients, but
greater sensitivity of some older individuals cannot be ruled out.
Metformin hydrochloride is known to be substantially excreted by the kidney and because the risk of
serious adverse reactions to the drug is greater in patients with impaired renal function,
glipizide/metformin HCl tablet should only be used in patients with normal renal function (see CONTRAINDICATIONS, WARNINGS, and CLINICAL PHARMACOLOGY:
Pharmacokinetics).
Because aging is associated with reduced renal function, glipizide/metformin HCl tablet should be
used with caution as age increases. Care should be taken in dose selection and should be based on
careful and regular monitoring of renal function. Generally, elderly patients should not be
titrated to the maximum dose of glipizide/metformin HCl tablet (see also WARNINGS and DOSAGE AND
ADMINISTRATION).
GLIPIZIDE AND METFORMIN HYDROCHLORIDE ADVERSE REACTIONS
Glipizide/Metformin HCl tablets
In a double-blind 24-week clinical trial involving glipizide/metformin HCl tablet as initial therapy, a total of 172 patients received glipizide/metformin HCl tablet 2.5 mg/250 mg, 173 received glipizide/metformin HCl tablet 2.5 mg/500 mg, 170 received glipizide, and 177 received metformin. The most common clinical adverse events in these treatment groups are listed in Table 4.
| Number (%) of Patients | ||||
|---|---|---|---|---|
| Adverse Event | Glipizide 5 mg tablets N=170 | Metformin 500 mg tablets N=177 | Glipizide/Metformin HCl Tablets 2.5 mg/250 mg tablets N=172 | Glipizide/Metformin HCl Tablets 2.5 mg/500 mg tablets N=173 |
| Upper respiratory infection | 12 (7.1) | 15 (8.5) | 17 (9.9) | 14 (8.1) |
| Diarrhea | 8 (4.7) | 15 (8.5) | 4 (2.3) | 9 (5.2) |
| Dizziness | 9 (5.3) | 2 (1.1) | 3 (1.7) | 9 (5.2) |
| Hypertension | 17 (10.0) | 10 (5.6) | 5 (2.9) | 6 (3.5) |
| Nausea/vomiting | 6 (3.5) | 9 (5.1) | 1 (0.6) | 3 (1.7) |
In a double-blind 18-week clinical trial involving glipizide/metformin HCl tablet as second-line therapy, a total of 87 patients received glipizide/metformin HCl tablet, 84 received glipizide, and 75 received metformin. The most common clinical adverse events in this clinical trial are listed in Table 5.
| Number (%) of Patients | |||||
|---|---|---|---|---|---|
| Adverse Event | Glipizide 5 mg tabletsa N=84 | Metformin 500 mg tabletsa N=75 | Glipizide/Metformin HCl Tablets 5 mg/500 mg tabletsa N=87 | ||
| a The dose of glipizide was fixed at 30 mg daily; doses of metformin and Glipizide/Metformin HCl tablets were titrated. | |||||
| Diarrhea | 11 (13.1) | 13 (17.3) | 16 (18.4) | ||
| Headache | 5 (6.0) | 4 (5.3) | 11 (12.6) | ||
| Upper respiratory infection | 11 (13.1) | 8 (10.7) | 9 (10.3) | ||
| Musculoskeletal pain | 6 (7.1) | 5 (6.7) | 7 (8.0) | ||
| Nausea/vomiting | 5 (6.0) | 6 (8.0) | 7 (8.0) | ||
| Abdominal pain | 7 (8.3) | 5 (6.7) | 5 (5.7) | ||
| UTI | 4 (4.8) | 6 (8.0) | 1 (1.1) | ||
Hypoglycemia
In a controlled initial therapy trial of glipizide/metformin HCl tablet 2.5 mg/250 mg and 2.5 mg/500 mg the numbers of patients with hypoglycemia documented by symptoms (such as dizziness, shakiness, sweating, and hunger) and a fingerstick blood glucose measurement ≤ 50 mg/dL were 5 (2.9%) for glipizide, 0 (0%) for metformin, 13 (7.6%) for glipizide/metformin HCl tablet 2.5 mg/250 mg, and 16 (9.3%) for glipizide/metformin HCl tablet 2.5 mg/500 mg. Among patients taking either glipizide/metformin HCl tablet 2.5 mg/250 mg or glipizide/metformin HCl tablet 2.5 mg/500 mg, nine (2.6%) patients discontinued glipizide/metformin HCl tablet due to hypoglycemic symptoms and one required medical intervention due to hypoglycemia. In a controlled second-line therapy trial of glipizide/metformin HCl tablet 5 mg/500 mg, the numbers of patients with hypoglycemia documented by symptoms and a fingerstick blood glucose measurement ≤ 50 mg/dL were 0 (0%) for glipizide, 1 (1.3%) for metformin, and 11 (12.6%) for glipizide/metformin HCl tablet. One (1.1%) patient discontinued glipizide/metformin HCl tablet therapy due to hypoglycemic symptoms and none required medical intervention due to hypoglycemia. (See PRECAUTIONS.)
Gastrointestinal Reactions
Among the most common clinical adverse events in the initial therapy trial were diarrhea and nausea/vomiting; the incidences of these events were lower with both glipizide/metformin HCl tablet dosage strengths than with metformin therapy. There were 4 (1.2%) patients in the initial therapy trial who discontinued glipizide/metformin HCl tablet therapy due to GI adverse events. Gastrointestinal symptoms of diarrhea, nausea/vomiting, and abdominal pain were comparable among glipizide/metformin HCl tablet, glipizide and metformin in the second-line therapy trial. There were 4 (4.6%) patients in the second-line therapy trial who discontinued glipizide/metformin HCl tablet therapy due to GI adverse events.
OVERDOSAGE
Glipizide
Overdosage of sulfonylureas, including glipizide, can produce hypoglycemia. Mild hypoglycemic symptoms, without loss of consciousness or neurological findings, should be treated aggressively with oral glucose and adjustments in drug dosage and/or meal patterns. Close monitoring should continue until the physician is assured that the patient is out of danger. Severe hypoglycemic reactions with coma, seizure, or other neurological impairment occur infrequently, but constitute medical emergencies requiring immediate hospitalization. If hypoglycemic coma is diagnosed or suspected, the patient should be given a rapid intravenous injection of concentrated (50%) glucose solution. This should be followed by a continuous infusion of a more dilute (10%) glucose solution at a rate that will maintain the blood glucose at a level above 100 mg/dL. Patients should be closely monitored for a minimum of 24 to 48 hours, since hypoglycemia may recur after apparent clinical recovery. Clearance of glipizide from plasma would be prolonged in persons with liver disease. Because of the extensive protein binding of glipizide, dialysis is unlikely to be of benefit.
Metformin Hydrochloride
Overdose of metformin hydrochloride has occurred, including ingestion of amounts greater than 50 grams. Hypoglycemia was reported in approximately 10% of cases, but no causal association with metformin hydrochloride has been established. Lactic acidosis has been reported in approximately 32% of metformin overdose cases (see WARNINGS). Metformin is dialyzable with a clearance of up to 170 mL/min under good hemodynamic conditions. Therefore, hemodialysis may be useful for removal of accumulated drug from patients in whom metformin overdosage is suspected.
GLIPIZIDE AND METFORMIN HYDROCHLORIDE DOSAGE AND ADMINISTRATION
General Considerations
Dosage of glipizide/metformin HCl Tablet must be individualized on the basis of both effectiveness and tolerance while not exceeding the maximum recommended daily dose of 20 mg glipizide/2000 mg metformin. Glipizide/metformin HCl tablet should be given with meals and should be initiated at a low dose, with gradual dose escalation as described below, in order to avoid hypoglycemia (largely due to glipizide), to reduce GI side effects (largely due to metformin), and to permit determination of the minimum effective dose for adequate control of blood glucose for the individual patient.
With initial treatment and during dose titration, appropriate blood glucose monitoring should be used to determine the therapeutic response to glipizide/metformin HCl tablet and to identify the minimum effective dose for the patient. Thereafter, HbA1c should be measured at intervals of approximately 3 months to assess the effectiveness of therapy. The therapeutic goal in all patients with type 2 diabetes is to decrease FPG, PPG, and HbA1c to normal or as near normal as possible. Ideally, the response to therapy should be evaluated using HbA1c (glycosylated hemoglobin), which is a better indicator of long-term glycemic control than FPG alone.
No studies have been performed specifically examining the safety and efficacy of switching to glipizide/metformin HCl tablet therapy in patients taking concomitant glipizide (or other sulfonylurea) plus metformin. Changes in glycemic control may occur in such patients, with either hyperglycemia or hypoglycemia possible. Any change in therapy of type 2 diabetes should be undertaken with care and appropriate monitoring.
Glipizide/Metformin HCl Tablet in Patients with Inadequate Glycemic Control on Diet and Exercise Alone
For patients with type 2 diabetes whose hyperglycemia cannot be satisfactorily managed with diet and exercise alone, the recommended starting dose of glipizide/metformin HCl tablet is 2.5 mg/250 mg once a day with a meal. For patients whose FPG is 280 mg/dL to 320 mg/dL a starting dose of glipizide/metformin HCl tablet 2.5 mg/500 mg twice daily should be considered. The efficacy of glipizide/metformin HCl tablet in patients whose FPG exceeds 320 mg/dL has not been established. Dosage increases to achieve adequate glycemic control should be made in increments of one tablet per day every two weeks up to maximum of 10 mg/1000 mg or 10 mg/2000 mg Glipizide/Metformin HCl tablets per day given in divided doses. In clinical trials of glipizide/metformin HCl tablet as initial therapy, there was no experience with total daily doses greater than 10 mg/2000 mg per day.
Glipizide/Metformin HCl Tablet in Patients with Inadequate Glycemic Control on a Sulfonylurea and/or Metformin
For patients not adequately controlled on either glipizide (or another sulfonylurea) or
metformin alone, the recommended starting dose of glipizide/metformin HCl tablet is 2.5 mg/500 mg
or 5 mg/500 mg twice daily with the morning and evening meals. In order to avoid hypoglycemia, the
starting dose of glipizide/metformin HCl tablet should not exceed the daily doses of glipizide or
metformin already being taken. The daily dose should be titrated in increments of no more than 5
mg/500 mg up to the minimum effective dose to achieve adequate control of blood glucose or to a
maximum dose of 20 mg/2000 mg per day.
Patients previously treated with combination
therapy of glipizide (or another sulfonylurea) plus metformin may be switched to
glipizide/metformin HCl tablet 2.5 mg/500 mg or 5 mg/500 mg; the starting dose should not exceed
the daily dose of glipizide (or equivalent dose of another sulfonylurea) and metformin already
being taken. The decision to switch to the nearest equivalent dose or to titrate should be based on
clinical judgment. Patients should be monitored closely for signs and symptoms of hypoglycemia
following such a switch and the dose of glipizide/metformin HCl tablet should be titrated as
described above to achieve adequate control of blood glucose.
Specific Patient Populations
Glipizide/metformin HCl tablet is not recommended for use during pregnancy or for use in pediatric patients. The initial and maintenance dosing of glipizide/metformin HCl tablet should be conservative in patients with advanced age, due to the potential for decreased renal function in this population. Any dosage adjustment requires a careful assessment of renal function. Generally, elderly, debilitated, and malnourished patients should not be titrated to the maximum dose of glipizide/metformin HCl tablet to avoid the risk of hypoglycemia. Monitoring of renal function is necessary to aid in prevention of metformin-associated lactic acidosis, particularly in the elderly. (See WARNINGS.)
HOW SUPPLIED
Glipizide/metformin HCl tablet 2.5 mg/250 mg is oval, peach colored, film coated tablets, debossed “727” on one side and ‘C’ on the other side and are available as follows:
Bottles of 100 NDC 57664-727-88
Bottles of 500 NDC 57664-727-13
Bottles of 1000 NDC 57664-727-18
Glipizide/metformin HCl tablet 2.5 mg/500 mg is oval, white to off-white, film coated tablets, debossed “725” on one side and ‘C’ on the other side and are available as follows:
Bottles of 100 NDC 57664-725-88
Bottles of 500 NDC 57664-725-13
Bottles of 1000 NDC 57664-725-18
Glipizide/metformin HCl tablet 5 mg/500 mg is oval, peach colored, film coated tablets, debossed “724” on one side and ‘C’ on the other side and are available as follows:
Bottles of 100 NDC 57664-724-88
Bottles of 500 NDC 57664-724-13
Bottles of 1000 NDC 57664-724-18
STORAGE
Store at 20° C to 25° C (68° F to 77° F); excursions permitted 15° C to 30° C (59° F to 86° F). [See USP Controlled Room Temperature.]
GLUCOPHAGE® (metformin hydrochloride tablets) is a registered trademark of Merck Santé S.A.S., an associate of Merck KGaA of Darmstadt, Germany. Licensed to Bristol-Myers Squibb Company.
GLUCOTROL® (glipizide) is a registered trademark of Pfizer Inc.
Manufactured by:
Caraco Pharmaceutical Laboratories, Ltd. C.S.No.: 5480T03
1150 Elijah McCoy Drive
Detroit, MI 48202
SUPPLEMENTAL PATIENT MATERIAL
PATIENT INFORMATION ABOUT
Glipizide/Metformin HCl Tablets
Glipizide and Metformin HydrochlorideGlipizide and Metformin Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Glipizide and Metformin HydrochlorideGlipizide and Metformin Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Glipizide and Metformin HydrochlorideGlipizide and Metformin Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||