GEMCITABINE HYDROCHLORIDE
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Gemcitabine for Injection, USP safely and effectively. See full prescribing information for Gemcitabine for Injection, USP.Gemcitabine for Injection, USP, Powder, Lyophilized, For Solution For Intravenous UseInitial U.S. Approval:1996 INDICATIONS AND USAGEGemcitabine for Injection, USP is a nucleoside metabolic inhibitor indicated for: Ovarian cancer in combination with carboplatin (1.1) Breast cancer in combination with paclitaxel (1.2) Non-small cell lung cancer in combination with cisplatin (1.3) Pancreatic cancer as a single-agent (1.4) DOSAGE AND ADMINISTRATIONGemcitabine is for intravenous use only. Ovarian cancer: 1000 mg/m2 over 30 minutes on Days 1 and 8 of each 21-day cycle (2.1) Breast cancer: 1250 mg/m2 over 30 minutes on Days 1 and 8 of each 21-day cycle (2.2) Non-small cell lung cancer: 4-week schedule, 1000 mg/m2 over 30 minutes on Days 1, 8, and 15 of each 28-day cycle: 3-week schedule; 1250 mg/m2 over 30 minutes on Days 1 and 8 of each 21-day cycle (2.3) Pancreatic cancer: 1000 mg/m2 over 30 minutes once weekly for up to 7 weeks (or until toxicity necessitates reducing or holding a dose), followed by a week of rest from treatment. Subsequent cycles should consist of infusions once weekly for 3 consecutive weeks out of every 4 weeks (2.4) Dose Reductions or discontinuation may be needed based on toxicities (2.1 to 2.4) DOSAGE FORMS AND STRENGTHS 200 mg vial for injection (3) 1 g vial for injection (3) 2 g vial for injection (3) CONTRAINDICATIONS4WARNINGS AND PRECAUTIONS Infusion time and dose frequency: Increased toxicity with infusion time >60 minutes or dosing more frequently than once weekly. (5.1) Hematology: Monitor for myelosuppression, which can be dose-limiting. (5.2, 5.7) Pulmonary toxicity: Discontinue gemcitabine immediately for severe pulmonary toxicity. (5.3) Renal: Monitor renal function prior to initiation of therapy and periodically thereafter. Use with caution in patients with renal impairment. Cases of hemolytic uremic syndrome (HUS) and/or renal failure, some fatal, have occurred. Discontinue gemcitabine for HUS or severe renal toxicity. (5.4) Hepatic: Monitor hepatic function prior to initiation of therapy and periodically thereafter. Use with caution in patients with hepatic impairment. Serious hepatotoxicity, including liver failure and death, have occurred. Discontinue gemcitabine for severe hepatic toxicity. (5.5) Pregnancy: Can cause fetal harm. Advise women of potential risk to the fetus. (5.6, 8.1) Radiation toxicity. May cause severe and life-threatening toxicity. (5.8) Side EffectsThe most common adverse reactions for the single-agent (≥ 20%) are nausea and vomiting, anemia, ALT, AST, neutropenia, leukopenia, alkaline phosphatase, proteinuria, fever, hematuria, rash, thrombocytopenia, dyspnea (6.1)To report SUSPECTED ADVERSE REACTIONS, contact APP Pharmaceuticals, LLC, Medical Affairs at 1-800-551-7176 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 INDICATIONS & USAGE
- 2 DOSAGE & ADMINISTRATION
- 3 DOSAGE FORMS & STRENGTHS
- 4 GEMCITABINE HYDROCHLORIDE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 GEMCITABINE HYDROCHLORIDE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 GEMCITABINE HYDROCHLORIDE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 15 REFERENCES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Gemcitabine 200mg-Vial Carton Label
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Gemcitabine 1g-Vial Carton Label
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Gemcitabine 2g-Vial Carton Label
FULL PRESCRIBING INFORMATION
1 INDICATIONS & USAGE
1.1 Ovarian Cancer
1.2 Breast Cancer
1.3 Non-Small Cell Lung Cancer
1.4 Pancreatic Cancer
2 DOSAGE & ADMINISTRATION
2.1 Ovarian Cancer
Gemcitabine for Injection should be administered intravenously at a dose of 1000 mg/m2 over 30 minutes on Days 1 and 8 of each 21-day cycle. Carboplatin AUC 4 should be administered intravenously on Day 1 after gemcitabine for injection administration. Patients should be monitored prior to each dose with a complete blood count, including differential counts. Patients should have an absolute granulocyte count ≥1500 x 106/L and a platelet count ≥100,000 x 106/L prior to each cycle.
Dose Modifications
Gemcitabine for Injection dosage adjustment for hematological toxicity within a cycle of treatment is based on the granulocyte and platelet counts taken on Day 8 of therapy. If marrow suppression is detected, gemcitabine for injection dosage should be modified according to guidelines in Table 1.
Table 1: Day 8 Dosage Reduction Guidelines for Gemcitabine for Injection in Combination with Carboplatin
| Absolute granulocyte count (x 106/L) |
Platelet count (x 106/L) |
% of full dose | |
|---|---|---|---|
| ≥ 1500 |
And |
≥ 100,000 |
100 |
| 1000 to 1499 |
and/or |
75,000 to 99,999 |
50 |
| < 1000 |
and/or |
< 75,000 |
Hold |
In general, for severe (Grade 3 or 4) non-hematological toxicity, except nausea/vomiting, therapy with gemcitabine for injection should be held or decreased by 50% depending on the judgment of the treating physician. For carboplatin dosage adjustment, see manufacturer’s prescribing information.
Dose adjustment for gemcitabine for injection in combination with carboplatin for subsequent cycles is based upon observed toxicity. The dose of gemcitabine for injection in subsequent cycles should be reduced to 800 mg/m2 on Days 1 and 8 in case of any of the following hematologic toxicities:
- Absolute granulocyte count <500 x 106/L for more than 5 days
- Absolute granulocyte count <100 x 106/L for more than 3 days
- Febrile neutropenia
- Platelets <25,000 x 106/L
- Cycle delay of more than one week due to toxicity
2.2 Breast Cancer
Gemcitabine for Injection should be administered intravenously at a dose of 1250 mg/m2 over 30 minutes on Days 1 and 8 of each 21-day cycle. Paclitaxel should be administered at 175 mg/m2 on Day 1 as a 3-hour intravenous infusion before gemcitabine administration. Patients should be monitored prior to each dose with a complete blood count, including differential counts. Patients should have an absolute granulocyte count ≥1500 x 106/L and a platelet count ≥100,000 x 106/L prior to each cycle.
Dose Modifications
Gemcitabine dosage adjustments for hematological toxicity is based on the granulocyte and platelet counts taken on Day 8 of therapy. If marrow suppression is detected, gemcitabine dosage should be modified according to the guidelines in Table 2.
Table 2: Day 8 Dosage Reduction Guidelines for Gemcitabine in Combination with Paclitaxel
| Absolute granulocyte count (x 106/L) |
Platelet count (x 106/L) |
% of full dose | |
|---|---|---|---|
| ≥ 1200 |
And |
> 75, 000 |
100 |
| 1000 to 1199 |
Or |
50,000 to 75,000 |
75 |
| 700 to 999 |
And |
≥ 50,000 |
50 |
| < 700 |
Or |
<50,000 |
Hold |
2.3 Non-Small Cell Lung Cancer
Two schedules have been investigated and the optimum schedule has not been determined [see Clinical Studies (14.3)]. With the 4-week schedule, gemcitabine should be administered intravenously at 1000 mg/m2 over 30 minutes on Days 1, 8, and 15 of each 28-day cycle. Cisplatin should be administered intravenously at 100 mg/m2 on Day 1 after the infusion of gemcitabine. With the 3-week schedule, gemcitabine should be administered intravenously at 1250 mg/m2 over 30 minutes on Days 1 and 8 of each 21-day cycle. Cisplatin at a dose of 100 mg/m2 should be administered intravenously after the infusion of gemcitabine on Day 1. See prescribing information for cisplatin administration and hydration guidelines.
Dose Modifications
Dosage adjustments for hematologic toxicity may be required for gemcitabine and for cisplatin. Gemcitabine dosage adjustment for hematological toxicity is based on the granulocyte and platelet counts taken on the day of therapy. Patients receiving gemcitabine should be monitored prior to each dose with a complete blood count (CBC), including differential and platelet counts. If marrow suppression is detected, therapy should be modified or suspended according to the guidelines in Table 3. For cisplatin dosage adjustment, see manufacturer’s prescribing information.
2.4 Pancreatic Cancer
Gemcitabine for Injection should be administered by intravenous infusion at a dose of 1000 mg/m2 over 30 minutes once weekly for up to 7 weeks (or until toxicity necessitates reducing or holding a dose), followed by a week of rest from treatment. Subsequent cycles should consist of infusions once weekly for 3 consecutive weeks out of every 4 weeks.
Dose Modifications
Dosage adjustment is based upon the degree of hematologic toxicity experienced by the patient [see Warnings and Precautions (5.2)]. Clearance in women and the elderly is reduced and women were somewhat less able to progress to subsequent cycles [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)].
Patients receiving gemcitabine should be monitored prior to each dose with a complete blood count (CBC), including differential and platelet count. If marrow suppression is detected, therapy should be modified or suspended according to the guidelines in Table 3.
Table 3: Dosage Reduction Guidelines
| Absolute granulocyte count (x106/L) |
Platelet count (x106/L) |
% of full dose | |
|---|---|---|---|
| ≥ 1000 |
And |
≥ 100,000 |
100 |
| 500 to 999 |
Or |
50,000 to 99,999 |
75 |
| < 500 |
Or |
< 50,000 |
Hold |
Laboratory evaluation of renal and hepatic function, including transaminases and serum creatinine, should be performed prior to initiation of therapy and periodically thereafter. Gemcitabine for Injection should be administered with caution in patients with evidence of significant renal or hepatic impairment as there is insufficient information from clinical studies to allow clear dose recommendation for these patient populations.
6666
2.5 Preparation and Administration Precautions
Caution should be exercised in handling and preparing gemcitabine solutions. The use of gloves is recommended. If gemcitabine solution contacts the skin or mucosa, immediately wash the skin thoroughly with soap and water or rinse the mucosa with copious amounts of water. Although acute dermal irritation has not been observed in animal studies, 2 of 3 rabbits exhibited drug-related systemic toxicities (death, hypoactivity, nasal discharge, shallow breathing) due to dermal absorption.
[see References (15)]2.6 Preparation for Intravenous Infusion Administration
The recommended diluent for reconstitution of gemcitabine is 0.9% Sodium Chloride Injection without preservatives. Due to solubility considerations, the maximum concentration for gemcitabine upon reconstitution is 40 mg/mL. Reconstitution at concentrations greater than 40 mg/mL may result in incomplete dissolution, and should be avoided.
When prepared as directed, gemcitabine solutions are stable for 24 hours at controlled room temperature 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Discard unused portion. Solutions of reconstituted gemcitabine should not be refrigerated, as crystallization may occur.
3 DOSAGE FORMS & STRENGTHS
Gemcitabine for Injection is a white to off-white lyophilized powder available in sterile single-use vials containing 200 mg, 1 g or 2 g gemcitabine.
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Infusion time
[see Clinical Studies (14.5 ) ]
5.2 Hematology
[see Adverse Reactions (6.1)], [see Dosage and Administration(2.1, 2.2, 2.3, and 2.4)]
5.3 Pulmonary
Pulmonary toxicity has been reported with the use of gemcitabine. In cases of severe lung toxicity, gemcitabine therapy should be discontinued immediately and appropriate supportive care measures instituted [see Adverse Reactions (6.1 and 6.2)].
5.4 Renal
Hemolytic Uremic Syndrome (HUS) and/or renal failure have been reported following one or more doses of gemcitabine. Renal failure leading to death or requiring dialysis, despite discontinuation of therapy, has been reported. The majority of the cases of renal failure leading to death were due to HUS [see Adverse Reactions (6.1 and 6.2)].
[see Use in Specific Populations (8.6)].5.5 Hepatic
Serious hepatotoxicity, including liver failure and death, has been reported in patients receiving gemcitabine alone or in combination with other potentially hepatotoxic drugs [see Adverse Reactions (6.1 and 6.2)].
[see Use in Specific Populations (8.7)].5.6 Pregnancy
[see Use in Specific Populations (8.1)].
5.7 Laboratory Tests
Patients receiving gemcitabine should be monitored prior to each dose with a complete blood count (CBC), including differential and platelet count. Suspension or modification of therapy should be considered when marrow suppression is detected [see Dosage and Administration (2.1, 2.2, 2.3, and 2.4)].
Laboratory evaluation of renal and hepatic function should be performed prior to initiation of therapy and periodically thereafter [see Dosage and Administration (2.4)].
5.8 Radiation Therapy
A pattern of tissue injury typically associated with radiation toxicity has been reported in association with concurrent and non-concurrent use of gemcitabine.
Non-concurrent (given >7 days apart) - Analysis of the data does not indicate enhanced toxicity when gemcitabine is administered more than 7 days before or after radiation, other than radiation recall. Data suggest that gemcitabine can be started after the acute effects of radiation have resolved or at least one week after radiation.
Concurrent (given together or ≤ 7 days apart)236 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Single-Agent Use:
[see Dosage and Administration (2.1, 2.2, 2.3, and 2.4)].
2
Table 4: Selected WHO-Graded Adverse Reactions in Patients Receiving Single-Agent Gemcitabine WHO Grades (% incidence)a
| |
All Patientsb
|
Pancreatic Cancer
Patientsc |
Discontinuations
(%)d |
||||
| All Grades |
Grade 3 |
Grade 4 |
All Grades |
Grade 3 |
Grade 4 |
All Patients |
|
|
Laboratorye
|
|
|
|
|
|
|
|
| Hematologic |
|
|
|
|
|
|
|
| Anemia |
68 |
7 |
1 |
73 |
8 |
2 |
<1 |
| Leukopenia |
62 |
9 |
<1 |
64 |
8 |
1 |
<1 |
| Neutropenia |
63 |
19 |
6 |
61 |
17 |
7 |
- |
| Thrombocytopenia |
24 |
4 |
1 |
36 |
7 |
<1 |
<1 |
| Hepatic |
|
|
|
|
|
|
<1 |
| ALT |
68 |
8 |
2 |
72 |
10 |
1 |
|
| AST |
67 |
6 |
2 |
78 |
12 |
5 |
|
| Alkaline Phosphatase |
55 |
7 |
2 |
77 |
16 |
4 |
|
| Bilirubin |
13 |
2 |
<1 |
26 |
6 |
2 |
|
| Renal |
|
|
|
|
|
|
<1 |
| Proteinuria |
45 |
<1 |
0 |
32 |
<1 |
0 |
|
| Hematuria |
35 |
<1 |
0 |
23 |
0 |
0 |
|
| BUN |
16 |
0 |
0 |
15 |
0 |
0 |
|
| Creatinine |
8 |
<1 |
0 |
6 |
0 |
0 |
|
|
Non-laboratoryf
|
|
|
|
|
|
|
|
| Nausea and Vomiting |
69 |
13 |
1 |
71 |
10 |
2 |
<1 |
| Fever |
41 |
2 |
0 |
38 |
2 |
0 |
<1 |
| Rash |
30 |
<1 |
0 |
28 |
<1 |
0 |
<1 |
| Dyspnea |
23 |
3 |
<1 |
10 |
0 |
<1 |
<1 |
| Diarrhea |
19 |
1 |
0 |
30 |
3 |
0 |
0 |
| Hemorrhage |
17 |
<1 |
<1 |
4 |
2 |
<1 |
<1 |
| Infection |
16 |
1 |
<1 |
10 |
2 |
<1 |
<1 |
| Alopecia |
15 |
<1 |
0 |
16 |
0 |
0 |
0 |
| Stomatitis |
11 |
<1 |
0 |
10 |
<1 |
0 |
<1 |
| Somnolence |
11 |
<1 |
<1 |
11 |
2 |
<1 |
<1 |
| Paresthesias |
10 |
<1 |
0 |
10 |
<1 |
0 |
0 |
a
b
c
d
e
f
Hematologic[see Dosage and Administration (2.1, 2.2, 2.3, and 2.4)]
Gastrointestinal
Hepatic[see Adverse Reactions (6.2)]
Renal[see Adverse Reactions (6.2)]
Fever
Rash
Pulmonary[see Adverse Reactions (6.2)]
Edema
Flu-like Symptoms
Infection
Alopecia
Neurotoxicity
Extravasation
Allergic [see Contraindications (4)]
Cardiovascular [see Adverse Reactions (6.2)]
Combination Use in Non-Small Cell Lung Cancer:
Table 5: Selected CTC-Graded Adverse Reactions From Comparative Trial of Gemcitabine Plus Cisplatin Versus Single-Agent Cisplatin in NSCLC CTC Grades (% incidence)a
| |
Gemcitabine plus Cisplatinb
|
Cisplatinc
|
||||
| All Grades |
Grade 3 |
Grade 4 |
All Grades |
Grade 3 |
Grade 4 |
|
|
Laboratoryd
|
|
|
|
|
|
|
| Hematologic |
|
|
|
|
|
|
| Anemia |
89 |
22 |
3 |
67 |
6 |
1 |
| RBC Transfusione
|
39 |
|
|
13 |
|
|
| Leukopenia |
82 |
35 |
11 |
25 |
2 |
1 |
| Neutropenia |
79 |
22 |
35 |
20 |
3 |
1 |
| Thrombocytopenia |
85 |
25 |
25 |
13 |
3 |
1 |
| Platelet Transfusionse
|
21 |
|
|
<1 |
|
|
| Lymphocytes |
75 |
25 |
18 |
51 |
12 |
5 |
| Hepatic |
|
|
|
|
|
|
| Transaminase |
22 |
2 |
1 |
10 |
1 |
0 |
| Alkaline Phosphatase |
19 |
1 |
0 |
13 |
0 |
0 |
| Renal |
|
|
|
|
|
|
| Proteinuria |
23 |
0 |
0 |
18 |
0 |
0 |
| Hematuria |
15 |
0 |
0 |
13 |
0 |
0 |
| Creatinine |
38 |
4 |
<1 |
31 |
2 |
<1 |
| Other Laboratory |
|
|
|
|
|
|
| Hyperglycemia |
30 |
4 |
0 |
23 |
3 |
0 |
| Hypomagnesemia |
30 |
4 |
3 |
17 |
2 |
0 |
| Hypocalcemia |
18 |
2 |
0 |
7 |
0 |
<1 |
|
Non-laboratoryf
|
|
|
|
|
|
|
| Nausea |
93 |
25 |
2 |
87 |
20 |
<1 |
| Vomiting |
78 |
11 |
12 |
71 |
10 |
9 |
| Alopecia |
53 |
1 |
0 |
33 |
0 |
0 |
| Neuro Motor |
35 |
12 |
0 |
15 |
3 |
0 |
| Neuro Hearing |
25 |
6 |
0 |
21 |
6 |
0 |
| Diarrhea |
24 |
2 |
2 |
13 |
0 |
0 |
| Neuro Sensory |
23 |
1 |
0 |
18 |
1 |
0 |
| Infection |
18 |
3 |
2 |
12 |
1 |
0 |
| Fever |
16 |
0 |
0 |
5 |
0 |
0 |
| Neuro Cortical |
16 |
3 |
1 |
9 |
1 |
0 |
| Neuro Mood |
16 |
1 |
0 |
10 |
1 |
0 |
| Local |
15 |
0 |
0 |
6 |
0 |
0 |
| Neuro Headache |
14 |
0 |
0 |
7 |
0 |
0 |
| Stomatitis |
14 |
1 |
0 |
5 |
0 |
0 |
| Hemorrhage |
14 |
1 |
0 |
4 |
0 |
0 |
| Dyspnea |
12 |
4 |
3 |
11 |
3 |
2 |
| Hypotension |
12 |
1 |
0 |
7 |
1 |
0 |
| Rash |
11 |
0 |
0 |
3 |
0 |
0 |
a
b2 2
c2
d
e
f
Table 6: Selected WHO-Graded Adverse Reactions From Comparative Trial of Gemcitabine Plus Cisplatin Versus Etoposide Plus Cisplatin in NSCLC WHO Grades (% incidence)a
| |
Gemcitabine plus Cisplatinb
|
Etoposide plus Cisplatinc
|
||||
| All Grades |
Grade 3 |
Grade 4 |
All Grades |
Grade 3 |
Grade 4 |
|
|
Laboratoryd
|
|
|
|
|
|
|
| Hematologic |
|
|
|
|
|
|
| Anemia |
88 |
22 |
0 |
77 |
13 |
2 |
| RBC Transfusionse
|
29 |
|
|
21 |
|
|
| Leukopenia |
86 |
26 |
3 |
87 |
36 |
7 |
| Neutropenia |
88 |
36 |
28 |
87 |
20 |
56 |
| Thrombocytopenia |
81 |
39 |
16 |
45 |
8 |
5 |
| Platelet Transfusionse
|
3 |
|
|
8 |
|
|
| Hepatic |
|
|
|
|
|
|
| ALT |
6 |
0 |
0 |
12 |
0 |
0 |
| AST |
3 |
0 |
0 |
11 |
0 |
0 |
| Alkaline Phosphatase |
16 |
0 |
0 |
11 |
0 |
0 |
| Bilirubin |
0 |
0 |
0 |
0 |
0 |
0 |
| Renal |
|
|
|
|
|
|
| Proteinuria |
12 |
0 |
0 |
5 |
0 |
0 |
| Hematuria |
22 |
0 |
0 |
10 |
0 |
0 |
| BUN |
6 |
0 |
0 |
4 |
0 |
0 |
| Creatinine |
2 |
0 |
0 |
2 |
0 |
0 |
|
Non-laboratory f,g
|
|
|
|
|
|
|
| Nausea and Vomiting |
96 |
35 |
4 |
86 |
19 |
7 |
| Fever |
6 |
0 |
0 |
3 |
0 |
0 |
| Rash |
10 |
0 |
0 |
3 |
0 |
0 |
| Dyspnea |
1 |
0 |
1 |
3 |
0 |
0 |
| Diarrhea |
14 |
1 |
1 |
13 |
0 |
2 |
| Hemorrhage |
9 |
0 |
3 |
3 |
0 |
3 |
| Infection |
28 |
3 |
1 |
21 |
8 |
0 |
| Alopecia |
77 |
13 |
0 |
92 |
51 |
0 |
| Stomatitis |
20 |
4 |
0 |
18 |
2 |
0 |
| Somnolence |
3 |
0 |
0 |
3 |
2 |
0 |
| Paresthesias |
38 |
0 |
0 |
16 |
2 |
0 |
a
b2 2
c22
d
e
f
g
Combination Use in Breast Cancer:
Table 7: Adverse Reactions From Comparative Trial of Gemcitabine Plus Paclitaxel Versus Single-Agent Paclitaxel in Breast Cancera CTC Grades (% incidence)
| |
Gemcitabine plus
Paclitaxel (N=262) |
Paclitaxel
(N=259) |
||||
| All Grades |
Grade 3 |
Grade 4 |
All Grades |
Grade 3 |
Grade 4 |
|
|
Laboratoryb
|
|
|
|
|
|
|
| Hematologic |
|
|
|
|
|
|
| Anemia |
69 |
6 |
1 |
51 |
3 |
<1 |
| Neutropenia |
69 |
31 |
17 |
31 |
4 |
7 |
| Thrombocytopenia |
26 |
5 |
<1 |
7 |
<1 |
<1 |
| Leukopenia |
21 |
10 |
1 |
12 |
2 |
0 |
| Hepatobiliary |
|
|
|
|
|
|
| ALT |
18 |
5 |
<1 |
6 |
<1 |
0 |
| AST |
16 |
2 |
0 |
5 |
<1 |
0 |
|
Non-laboratoryc
|
|
|
|
|
|
|
| Alopecia |
90 |
14 |
4 |
92 |
19 |
3 |
| Neuropathy-sensory |
64 |
5 |
<1 |
58 |
3 |
0 |
| Nausea |
50 |
1 |
0 |
31 |
2 |
0 |
| Fatigue |
40 |
6 |
<1 |
28 |
1 |
<1 |
| Myalgia |
33 |
4 |
0 |
33 |
3 |
<1 |
| Vomiting |
29 |
2 |
0 |
15 |
2 |
0 |
| Arthralgia |
24 |
3 |
0 |
22 |
2 |
<1 |
| Diarrhea |
20 |
3 |
0 |
13 |
2 |
0 |
| Anorexia |
17 |
0 |
0 |
12 |
<1 |
0 |
| Neuropathy-motor |
15 |
2 |
<1 |
10 |
<1 |
0 |
| Stomatitis/pharyngitis |
13 |
1 |
<1 |
8 |
<1 |
0 |
| Fever |
13 |
<1 |
0 |
3 |
0 |
0 |
| Rash/desquamation |
11 |
<1 |
<1 |
5 |
0 |
0 |
a
b
c
Combination Use in Ovarian Cancer:
Table 8: Adverse Reactions From Comparative Trial of Gemcitabine Plus Carboplatin Versus Single-Agent Carboplatin in Ovarian Cancera CTC Grades (% incidence)
| |
Gemcitabine plus Carboplatin
(N=175) |
Carboplatin
(N=174) |
||||
| All Grades |
Grade 3 |
Grade 4 |
All Grades |
Grade 3 |
Grade 4 |
|
|
Laboratoryb
|
|
|
|
|
|
|
| Hematologic |
|
|
|
|
|
|
| Neutropenia |
90 |
42 |
29 |
58 |
11 |
1 |
| Anemia |
86 |
22 |
6 |
75 |
9 |
2 |
| Leukopenia |
86 |
48 |
5 |
70 |
6 |
<1 |
| Thrombocytopenia |
78 |
30 |
5 |
57 |
10 |
1 |
| RBC Transfusionsc
|
38 |
|
|
15 |
|
|
| Platelet Transfusionsc
|
9 |
|
|
3 |
|
|
|
Non-laboratoryb
|
|
|
|
|
|
|
| Nausea |
69 |
6 |
0 |
61 |
3 |
0 |
| Alopecia |
49 |
0 |
0 |
17 |
0 |
0 |
| Vomiting |
46 |
6 |
0 |
36 |
2 |
<1 |
| Constipation |
42 |
6 |
1 |
37 |
3 |
0 |
| Fatigue |
40 |
3 |
<1 |
32 |
5 |
0 |
| Neuropathy-sensory |
29 |
1 |
0 |
27 |
2 |
0 |
| Diarrhea |
25 |
3 |
0 |
14 |
<1 |
0 |
| Stomatitis/pharyngitis |
22 |
<1 |
0 |
13 |
0 |
0 |
| Anorexia |
16 |
1 |
0 |
13 |
0 |
0 |
a
b
c
6.2 Post-Marketing Experience
The following adverse reactions have been identified during post-approval use of gemcitabine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
These adverse reactions have occurred after gemcitabine single-agent use and gemcitabine in combination with other cytotoxic agents. Decisions to include these events are based on the seriousness of the event, frequency of reporting, or potential causal connection to gemcitabine.
Cardiovascular - Congestive heart failure and myocardial infarction have been reported very rarely with the use of gemcitabine. Arrhythmias, predominantly supraventricular in nature, have been reported very rarely.
Vascular Disorders - Clinical signs of peripheral vasculitis and gangrene have been reported very rarely.
Skin - Cellulitis and non-serious injection site reactions in the absence of extravasation have been rarely reported. Severe skin reactions, including desquamation and bullous skin eruptions, have been reported very rarely.
Hepatic - Increased liver function tests including elevations in aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), alkaline phosphatase, and bilirubin levels have been reported rarely. Serious hepatotoxicity including liver failure and death has been reported very rarely in patients receiving gemcitabine alone or in combination with other potentially hepatotoxic drugs. Hepatic veno-occlusive disease has been reported.
Pulmonary - Parenchymal toxicity, including interstitial pneumonitis, pulmonary fibrosis, pulmonary edema, and adult respiratory distress syndrome (ARDS), has been reported rarely following one or more doses of gemcitabine administered to patients with various malignancies. Some patients experienced the onset of pulmonary symptoms up to 2 weeks after the last gemcitabine dose. Respiratory failure and death occurred very rarely in some patients despite discontinuation of therapy.
Renal - Hemolytic Uremic Syndrome (HUS) and/or renal failure have been reported following one or more doses of gemcitabine. Renal failure leading to death or requiring dialysis, despite discontinuation of therapy, has been rarely reported. The majority of the cases of renal failure leading to death were due to HUS.
Injury, Poisoning, and Procedural Complications[see Warnings and Precautions (5.8)]7 DRUG INTERACTIONS
[see Clinical Pharmacology (12.2 and 12.3)]
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category D [see Warnings and Precautions (5.6) ]
22[see Warnings and Precautions (5.6)]8.3 Nursing Mothers
8.4 Pediatric Use
22
8.5 Geriatric Use
[see Clinical Pharmacology (12.3)][see Dosage and Administration (2.1, 2.2, 2.3, and 2.4)][see Clinical Studies (14.1)]
8.6 Renal
Hemolytic Uremic Syndrome (HUS) and/or renal failure have been reported following one or more doses of gemcitabine. Renal failure leading to death or requiring dialysis, despite discontinuation of therapy, has been reported. The majority of the cases of renal failure leading to death were due to HUS [see Adverse Reactions (6.1 and 6.2)].
[see Warnings and Precautions (5.4)]8.7 Hepatic
Serious hepatotoxicity, including liver failure and death, has been reported in patients receiving gemcitabine alone or in combination with other potentially hepatotoxic drugs [see Adverse Reactions (6.1 and 6.2)].
[see Warnings and Precautions (5.5) ]8.8 Gender
[see Clinical Pharmacology (12.3)].[see Dosage and Administration (2)]
10 OVERDOSAGE
2
11 DESCRIPTION
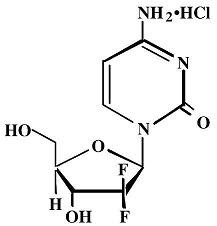
Gemcitabine for Injection is a nucleoside metabolic inhibitor that exhibits antitumor activity. Gemcitabine HCl is 2´-deoxy-2´,2´-difluorocytidine monohydrochloride (β-isomer).
911234
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Gemcitabine exhibits cell phase specificity, primarily killing cells undergoing DNA synthesis (S-phase) and also blocking the progression of cells through the G1/S-phase boundary. Gemcitabine is metabolized intracellularly by nucleoside kinases to the active diphosphate (dFdCDP) and triphosphate (dFdCTP) nucleosides. The cytotoxic effect of gemcitabine is attributed to a combination of two actions of the diphosphate and the triphosphate nucleosides, which leads to inhibition of DNA synthesis. First, gemcitabine diphosphate inhibits ribonucleotide reductase, which is responsible for catalyzing the reactions that generate the deoxynucleoside triphosphates for DNA synthesis. Inhibition of this enzyme by the diphosphate nucleoside causes a reduction in the concentrations of deoxynucleotides, including dCTP. Second, gemcitabine triphosphate competes with dCTP for incorporation into DNA. The reduction in the intracellular concentration of dCTP (by the action of the diphosphate) enhances the incorporation of gemcitabine triphosphate into DNA (self-potentiation). After the gemcitabine nucleotide is incorporated into DNA, only one additional nucleotide is added to the growing DNA strands. After this addition, there is inhibition of further DNA synthesis. DNA polymerase epsilon is unable to remove the gemcitabine nucleotide and repair the growing DNA strands (masked chain termination). In CEM T lymphoblastoid cells, gemcitabine induces internucleosomal DNA fragmentation, one of the characteristics of programmed cell death.
12.2 Pharmacodynamics
in vitroIn vivo
12.3 Pharmacokinetics
Absorption and Distribution
The pharmacokinetics of gemcitabine were examined in 353 patients, with various solid tumors. Pharmacokinetic parameters were derived using data from patients treated for varying durations of therapy given weekly with periodic rest weeks and using both short infusions (<70 minutes) and long infusions (70 to 285 minutes). The total gemcitabine dose varied from 500 to 3600 mg/m2.
The volume of distribution was increased with infusion length. Volume of distribution of gemcitabine was 50 L/m2 following infusions lasting <70 minutes. For long infusions, the volume of distribution rose to 370 L/m2.
Gemcitabine pharmacokinetics are linear and are described by a 2-compartment model. Population pharmacokinetic analyses of combined single and multiple dose studies showed that the volume of distribution of gemcitabine was significantly influenced by duration of infusion and gender. Gemcitabine plasma protein binding is negligible.
Metabolism
Gemcitabine disposition was studied in 5 patients who received a single 1000 mg/m2/30 minute infusion of radiolabeled drug. Within one (1) week, 92% to 98% of the dose was recovered, almost entirely in the urine. Gemcitabine (<10%) and the inactive uracil metabolite, 2´-deoxy-2´,2´-difluorouridine (dFdU), accounted for 99% of the excreted dose. The metabolite dFdU is also found in plasma.
The active metabolite, gemcitabine triphosphate, can be extracted from peripheral blood mononuclear cells. The half-life of the terminal phase for gemcitabine triphosphate from mononuclear cells ranges from 1.7 to 19.4 hours.
Excretion
Clearance of gemcitabine was affected by age and gender. The lower clearance in women and the elderly results in higher concentrations of gemcitabine for any given dose. Differences in either clearance or volume of distribution based on patient characteristics or the duration of infusion result in changes in half-life and plasma concentrations. Table 9 shows plasma clearance and half-life of gemcitabine following short infusions for typical patients by age and gender.
Table 9: Gemcitabine Clearance and Half-Life for the “Typical” Patient
| Age | Clearance Men (L/hr/m2) |
Clearance Women (L/hr/m2) |
Half-Lifea
Men (min) |
Half-Lifea
Women (min) |
|---|---|---|---|---|
|
a Half-life for patients receiving a short infusion (<70 min). |
||||
| 29 |
92.2 |
69.4 |
42 |
49 |
| 45 |
75.7 |
57 |
48 |
57 |
| 65 |
55.1 |
41.5 |
61 |
73 |
| 79 |
40.7 |
30.7 |
79 |
94 |
Gemcitabine half-life for short infusions ranged from 42 to 94 minutes, and the value for long infusions varied from 245 to 638 minutes, depending on age and gender, reflecting a greatly increased volume of distribution with longer infusions.
Drug Interactions
22222[see Drug Interactions (7)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
in vitro in vivo in vivo in vitro in vitro2 2 2
14 CLINICAL STUDIES
14.1 Ovarian Cancer
There was not a significant difference in overall survival between arms.
Table 10: Gemcitabine Plus Carboplatin Versus Carboplatin in Ovarian Cancer - Baseline Demographics and Clinical Characteristics
| Gemcitabine/ Carboplatin |
Carboplatin | |
|---|---|---|
|
a Nine patients (5 on the gemcitabine plus carboplatin arm and 4 on the carboplatin arm) did not have baseline Eastern Cooperative Oncology Group (ECOG) performance status recorded. |
||
| Number of randomized patients | 178 |
178 |
| Median age, years Range |
59 36 to 78 |
58 21 to 81 |
| Baseline ECOG performance status 0-1a | 94% |
95% |
| Disease Status Evaluable Bidimensionally measurable |
7.9% 91.6% |
2.8% 95.5% |
| Platinum-free intervalb
6 to 12 months >12 months |
39.9% 59% |
39.9% 59.6% |
| First-line therapy Platinum-taxane combination Platinum-non-taxane combination Platinum monotherapy |
70.2% 28.7% 1.1% |
71.3% 27.5% 1.1% |
b
Table 11: Gemcitabine Plus Carboplatin Versus Carboplatin in Ovarian Cancer - Results of Efficacy Analysis
| Gemcitabine/Carboplatin (N=178) |
Carboplatin (N=178) |
||
|---|---|---|---|
| PFS |
|
|
p=0.0038d
|
| Median (95%, C.I.) months
|
8.6 (8, 9.7) |
5.8 (5.2, 7.1) |
|
| Hazard Ratio (95%, C.I.)
|
0.72 (0.57, 0.90) |
|
|
| Overall Survival |
|
|
p=0.8977d
|
| Median (95%, C.I.) Months |
18 (16.2, 20.3) |
17.3 (15.2, 19.3) |
|
| Hazard Ratio (95%, C.I.) |
0.98 (0.78, 1.24) |
|
|
| Adjusteda Hazard Ratio (95%, C.I.) |
0.86 (0.67, 1.1) |
|
|
| Investigator Reviewed |
|
|
p=0.0016e
|
| Overall Response Rate |
47.2% |
30.9% |
|
| CR |
14.6% |
6.2% |
|
| PR+PRNMb
|
32.6% |
24.7% |
|
| Independently Reviewed |
|
|
p=0.11e |
| Overall Response Ratec,f
|
46.3% |
35.6% |
|
| CR |
9.1% |
4% |
|
| PR+PRNM |
37.2% |
31.7% |
|
aTreatment adjusted for performance status, tumor area, and platinum-free interval.
b Partial response non-measurable disease
c Independent reviewers could not evaluate disease demonstrated by sonography or physical exam.
d Log Rank, unadjusted
e Chi Square
f Independently reviewed cohort – Gemcitabine /Carboplatin N=121, Carboplatin N=101
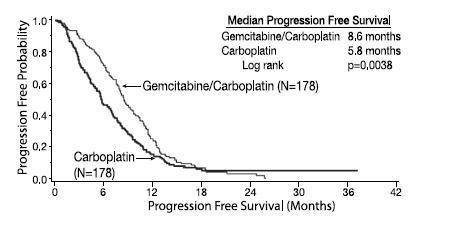
Figure 1: Kaplan-Meier Curve of Progression Free Survival in Gemcitabine for Injection Plus Carboplatin Versus Carboplatin in Ovarian Cancer (N=356)
14.2 Breast Cancer
Data from a multi-national, randomized Phase 3 study (529 patients) support the use of gemcitabine in combination with paclitaxel for treatment of breast cancer patients who have received prior adjuvant/neoadjuvant anthracycline chemotherapy unless clinically contraindicated. Gemcitabine 1250 mg/m2 was administered on Days 1 and 8 of a 21-day cycle with paclitaxel 175 mg/m2 administered prior to gemcitabine on Day 1 of each cycle. Single-agent paclitaxel 175 mg/m2 was administered on Day 1 of each 21-day cycle as the control arm.
The addition of gemcitabine to paclitaxel resulted in statistically significant improvement in time to documented disease progression and overall response rate compared to monotherapy with paclitaxel as shown in Table 12 and Figure 2. Final survival analysis results at 440 events were Hazard Ratio of 0.86 (95%, CI: 0.71 – 1.04) for the ITT population, as shown in Table 12.
Table 12: Gemcitabine Plus Paclitaxel Versus Paclitaxel in Breast Cancer
| Gemcitabine/ Paclitaxel |
Paclitaxel | ||
|---|---|---|---|
|
a Karnofsky Performance Status. b Based on the ITT population c These represent reconciliation of investigator and Independent Review Committee assessments according to a predefined algorithm. |
|||
| Number of Patients |
267 |
262 |
|
| Median age, years Range |
53 26 to 83 |
52 26 to 75 |
|
| Metastatic disease |
97% |
96.9% |
|
| Baseline KPSa
> 90 |
70.4 % |
74.4% |
|
| Number of tumor sites 1 to 2 >3 |
56.6% 43.4% |
58.8% 41.2% |
|
| Visceral disease |
73.4% |
72.9% |
|
| Prior anthracycline |
96.6% |
95.8% |
|
| Overall Survivalb
|
|
|
|
| Median (95 %, CI) |
18.6 (16.5, 20.7) |
15.8 (14.1, 17.3) |
|
| Hazard Ratio (95%, CI) |
0.86 (0.71, 1.04) |
|
|
| Time to Documented Disease Progressionc Median (95%, C.I.), months |
5.2 (4.2, 5.6) |
2.9 (2.6, 3.7) |
p<0.0001 |
| Hazard Ratio (95%, C.I.) |
0.65 (0.524, 0.805) |
p<0.0001 |
|
| Overall Response Ratec (95%, C.I.) | 40.8% (34.9, 46.7) |
22.1% (17.1, 27.2) |
p<0.0001 |
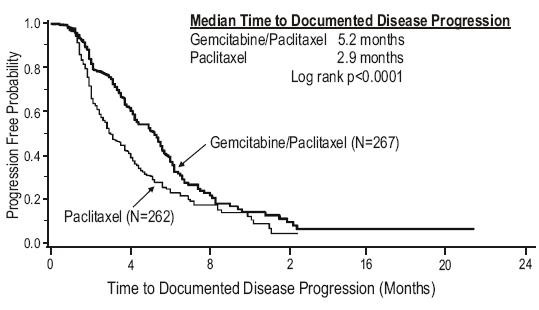
Figure 2: Kaplan-Meier Curve of Time to Documented Disease Progression in Gemcitabine Plus Paclitaxel Versus Paclitaxel Breast Cancer Study (N=529).
14.3 Non-Small Cell Lung Cancer (NSCLC)
Data from 2 randomized clinical studies (657 patients) support the use of gemcitabine in combination with cisplatin for the first-line treatment of patients with locally advanced or metastatic NSCLC.
Gemcitabine plus cisplatin versus cisplatin: This study was conducted in Europe, the US, and Canada in 522 patients with inoperable Stage IIIA, IIIB, or IV NSCLC who had not received prior chemotherapy. Gemcitabine 1000 mg/m2 was administered on Days 1, 8, and 15 of a 28-day cycle with cisplatin 100 mg/m2 administered on Day 1 of each cycle. Single-agent cisplatin 100 mg/m2 was administered on Day 1 of each 28-day cycle. The primary endpoint was survival. Patient demographics are shown in Table 13. An imbalance with regard to histology was observed with 48% of patients on the cisplatin arm and 37% of patients on the gemcitabine plus cisplatin arm having adenocarcinoma.
The Kaplan-Meier survival curve is shown in Figure 3. Median survival time on the gemcitabine plus cisplatin arm was 9 months compared to 7.6 months on the single-agent cisplatin arm (Log rank p=0.008, two-sided). Median time to disease progression was 5.2 months on the gemcitabine plus cisplatin arm compared to 3.7 months on the cisplatin arm (Log rank p=0.009, two-sided). The objective response rate on the gemcitabine plus cisplatin arm was 26% compared to 10% with cisplatin (Fisher’s Exact p<0.0001, two-sided). No difference between treatment arms with regard to duration of response was observed.
Gemcitabine plus cisplatin versus etoposide plus cisplatin: A second, multicenter, study in Stage IIIB or IV NSCLC randomized 135 patients to gemcitabine 1250 mg/m2 on Days 1 and 8, and cisplatin 100 mg/m2 on Day 1 of a 21-day cycle or to intravenous etoposide 100 mg/m2 on Days 1, 2, and 3 and cisplatin 100 mg/m2 on Day 1 of a 21-day cycle (Table 13).
There was no significant difference in survival between the two treatment arms (Log rank p=0.18, two-sided). The median survival was 8.7 months for the gemcitabine plus cisplatin arm versus 7 months for the etoposide plus cisplatin arm. Median time to disease progression for the gemcitabine plus cisplatin arm was 5 months compared to 4.1 months on the etoposide plus cisplatin arm (Log rank p=0.015, two-sided). The objective response rate for the gemcitabine plus cisplatin arm was 33% compared to 14% on the etoposide plus cisplatin arm (Fisher’s Exact p=0.01, two-sided).
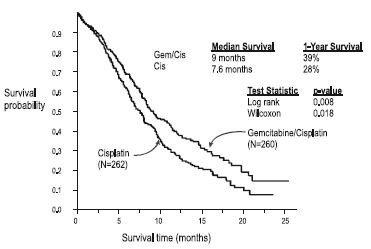
Figure 3: Kaplan-Meier Survival Curve in Gemcitabine Plus Cisplatin Versus Cisplatin NSCLC Study (N=522)
Table 13: Randomized Trials of Combination Therapy With Gemcitabine Plus Cisplatin in NSCLC
| Trial | 28-day Schedulea | 21-day Scheduleb | ||||
|---|---|---|---|---|---|---|
| Treatment Arm |
Gemcitabine/ Cisplatin |
Cisplatin |
|
Gemcitabine/ Cisplatin |
Cisplatin/ Etoposide |
|
| Number of patients Male Female |
260 182 78 |
262 186 76 |
69 64 5 |
66 61 5 |
||
| Median age, years Range |
62 36 to 88 |
63 35 to 79 |
58 33 to 76 |
60 35 to 75 |
||
| Stage IIIA Stage IIIB Stage IV |
7% 26% 67% |
7% 23% 70% |
N/Ac
48% 52% |
N/Ac
52% 49% |
||
| Baseline KPSd 70 to 80 Baseline KPSd 90 to 100 |
41% 57% |
44% 55% |
45% 55% |
52% 49% |
||
| Survival Median, months (95%, C.I.) months |
9 8.2, 11 |
7.6 6.6, 8.8 |
p=0.008 |
8.7 7.8, 10.1 |
7 6, 9.7 |
p=0.18 |
| Time to Disease Progression Median, months (95%, C.I.) months |
5.2 4.2, 5.7 |
3.7 3, 4.3 |
p=0.009 |
5 4.2, 6.4 |
4.1 2.4, 4.5 |
p=0.015 |
| Tumor Response | 26% |
10% |
p<0.0001e
|
33% |
14% |
p=0.01e
|
a 28-day schedule — Gemcitabine plus cisplatin:gemcitabine 1000 mg/m2 on Days 1, 8, and 15 and cisplatin 100 mg/m2 on Day 1 every 28 days;
Single-agent cisplatin: cisplatin 100 mg/m2 on Day 1 every 28 days.
b 21-day schedule — Gemcitabine plus cisplatin: gemcitabine 1250 mg/m2 on Days 1 and 8 and cisplatin 100 mg/m2 on Day 1 every 21 days;
Etoposide plus Cisplatin: cisplatin 100 mg/m2 on Day 1 and intravenous etoposide 100 mg/m2 on Days 1, 2, and 3 every 21 days.
c N/A Not applicable.
d Karnofsky Performance Status.
e p-value for tumor response was calculated using the two-sided Fisher’s Exact test for difference in binomial proportions.
All other p-values were calculated using the Log rank test for difference in overall time to an event.
14.4 Pancreatic Cancer
Data from 2 clinical trials evaluated the use of gemcitabine in patients with locally advanced or metastatic pancreatic cancer. The first trial compared gemcitabine to 5-Fluorouracil (5-FU) in patients who had received no prior chemotherapy. A second trial studied the use of gemcitabine in pancreatic cancer patients previously treated with 5-FU or a 5-FU-containing regimen. In both studies, the first cycle of gemcitabine was administered intravenously at a dose of 1000 mg/m2 over 30 minutes once weekly for up to 7 weeks (or until toxicity necessitated holding a dose) followed by a week of rest from treatment with gemcitabine. Subsequent cycles consisted of injections once weekly for 3 consecutive weeks out of every 4 weeks.
>
OR:
ii) the patient was stable on all of the aforementioned parameters, and showed marked, sustained weight gain (>7% increase maintained for > 4weeks) not due to fluid accumulation.
The first study was a multicenter (17 sites in US and Canada), prospective, single-blinded, two-arm, randomized, comparison of gemcitabine and 5-FU in patients with locally advanced or metastatic pancreatic cancer who had received no prior treatment with chemotherapy. 5-FU was administered intravenously at a weekly dose of 600 mg/m2 for 30 minutes. The results from this randomized trial are shown in Table 14. Patients treated with gemcitabine had statistically significant increases in clinical benefit response, survival, and time to disease progression compared to 5-FU. The Kaplan-Meier curve for survival is shown in Figure 4. No confirmed objective tumor responses were observed with either treatment.
Table 14: Gemcitabine Versus 5-FU in Pancreatic Cancer
| Gemcitabine | 5-FU | ||
|---|---|---|---|
|
a Karnofsky Performance Status. b Kaplan-Meier estimates. c N=number of patients. d No progression at last visit; remains alive. e The p-value for clinical benefit response was calculated using the two-sided test for difference in binomial proportions. All other p-values were calculated using the Log rank test for difference in overall time to an event. |
|||
| Number of patients Male Female |
63 34 29 |
63 34 29 |
|
| Median age Range |
62 years 37 to 79 |
61 years 36 to 77 |
|
| Stage IV disease | 71.4% |
76.2% |
|
| Baseline KPSa <70 | 69.8% |
68.3% |
|
| Clinical benefit response | 22.2% (Nc=14) |
4.8% (Nc=3) |
p=0.004e
|
| Survival Median 6-month probabilityb 9-month probabilityb 1-year probabilityb Range 95% C.I. of the median |
5.7 months (N=30) 46% (N=14) 24% (N=9) 18% 0.2 to 18.6 months 4.7 to 6.9 months |
4.2 months (N=19) 29% (N=4) 5% (N=2) 2% 0.4 to 15.1 +d months 3.1 to 5.1 months |
p=0.0009 |
| Time to Disease Progression Median Range 95% C.I. of the median |
2.1 months 0.1+d to 9.4 months 1.9 to 3.4 months |
0.9 months 0.1 to 12 +d months 0.9 to 1.1 months |
p=0.0013 |
Clinical benefit response was achieved by 14 patients treated with gemcitabine and 3 patients treated with 5-FU. One patient on the gemcitabine arm showed improvement in all 3 primary parameters (pain intensity, analgesic consumption, and performance status).
Eleven patients on the gemcitabine arm and 2 patients on the 5-FU arm showed improvement in analgesic consumption and/or pain intensity with stable performance status. Two patients on the gemcitabine arm showed improvement in analgesic consumption or pain intensity with improvement in performance status. One patient on the 5-FU arm was stable with regard to pain intensity and analgesic consumption with improvement in performance status. No patient on either arm achieved a clinical benefit response based on weight gain.
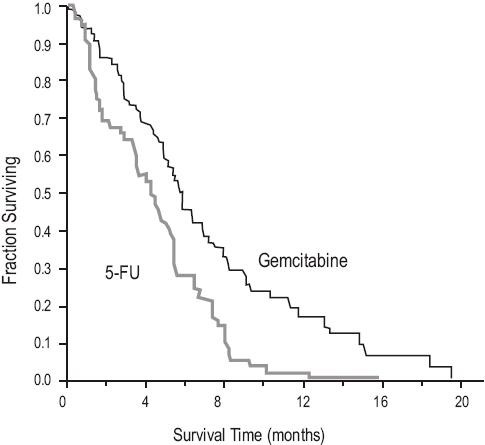
Figure 4: Kaplan-Meier Survival Curve
14.5 Other Clinical Studies
22 2 2 [see Clinical Pharmacology (12.3)] [see Warnings and Precautions (5.1) ]
15 REFERENCES
- NIOSH Alert: Preventing occupational exposures to antineoplastic and other hazardous drugs in healthcare settings. 2004. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2004-165.
- OSHA Technical Manual, TED 1-0.15A, Section VI: Chapter 2. Controlling Occupational Exposure to Hazardous Drugs. OSHA, 1999. http://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2.html
- American Society of Health-System Pharmacists. ASHP Guidelines on Handling Hazardous Drugs: Am J Health-Syst Pharm. 2006;63:1172-1193.
- Polovich, M., White, J. M., & Kelleher, L. O. (eds.) 2005. Chemotherapy and biotherapy guidelines and recommendations for practice (2nd. ed.) Pittsburgh, PA: Oncology Nursing Society.
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
|
Product
No. |
NDC
No. |
Strength |
|
| FK101210 |
63323-102-13 |
200 mg/vial |
10 mL single use vial packaged individually. |
| FK102550 |
63323-125-53 |
1 gram/vial |
50 mL single use vial packaged individually. |
| FK102600 |
63323-126-03 |
2 gram/vial |
100 mL single use vial packaged individually. |
16.2 Storage and Handling
[see Dosage and Administration (2.5 and 2.6)].
17 PATIENT COUNSELING INFORMATION
17.1 Low Blood Cell Counts
[see Warnings and Precautions (5.2)]
17.2 Pregnancy
[see Warnings and Precautions (5.6) and Use in Specific Populations (8.1)].
17.3 Nursing Mothers
[see Use in Specific Populations (8.3)]

APP Pharmaceuticals, LLC
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Gemcitabine 200mg-Vial Carton Label
GEMCITABINE
FOR INJECTION, USP
For IV use only
Must Be Diluted
DO NOT REFRIGERATE

PACKAGE LABEL - PRINCIPAL DISPLAY - Gemcitabine 200 mg-Vial Label
GEMCITABINE
FOR INJECTION, USP
For IV use only
Must Be Diluted

PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Gemcitabine 1g-Vial Carton Label
GEMCITABINE
FOR INJECTION, USP
For IV use only
Must Be Diluted
DO NOT REFRIGERATE

PACKAGE LABEL - PRINCIPAL DISPLAY - Gemcitabine 1 g-Vial Label
GEMCITABINE
FOR INJECTION, USP
For IV use only
Must Be Diluted
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Gemcitabine 2g-Vial Carton Label
GEMCITABINE
FOR INJECTION, USP
For IV use only
Must Be Diluted
DO NOT REFRIGERATE
PACKAGE LABEL - PRINCIPAL DISPLAY - Gemcitabine 2 g-Vial Label
GEMCITABINE
FOR INJECTION, USP
For IV use only
Must Be Diluted

GEMCITABINE HYDROCHLORIDEGEMCITABINE INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
GEMCITABINE HYDROCHLORIDEGEMCITABINE INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
GEMCITABINE HYDROCHLORIDEGEMCITABINE INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||