Gabapentin
FULL PRESCRIBING INFORMATION: CONTENTS*
- GABAPENTIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- GABAPENTIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- GABAPENTIN ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- MEDICATION GUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
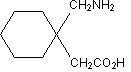
GABAPENTIN DESCRIPTION
CLINICAL PHARMACOLOGY
Mechanism of ActionPharmacokinetics and Drug Metabolism
All pharmacological actions following gabapentin administration are due to the activity of the parent compound; gabapentin is not appreciably metabolized in humans.
Special Populations: Patients With Renal Insufficiency, below). In elderly patients, and in patients with impaired renal function, gabapentin plasma clearance is reduced. Gabapentin can be removed from plasma by hemodialysis.
Dosage adjustment in patients with compromised renal function or undergoing hemodialysis is recommended (seeDOSAGE AND ADMINISTRATION, Table 6).
Special Populations
DOSAGE AND ADMINISTRATION). Pediatric patients with renal insufficiency have not been studied.
DOSAGE AND ADMINISTRATION).
(See PRECAUTIONS, Geriatric Use, andDOSAGE AND ADMINISTRATION.)
DOSAGE AND ADMINISTRATION).
Clinical Studies
TABLE 1. Controlled PHN Studies: Duration, Dosages, and Number of Patients
Study Study
Duration Gabapentin (mg/day)a
Target Dose Patients Receiving
Gabapentin Patients Receiving
Placebo
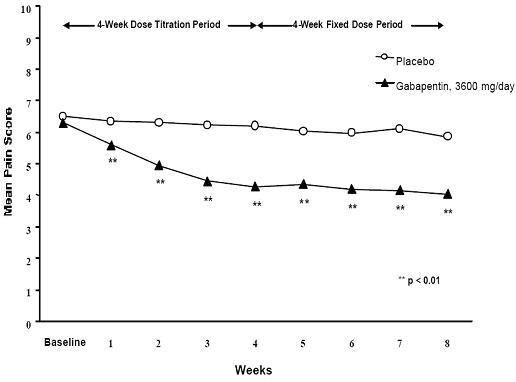
Figure 1. Weekly Mean Pain Scores (Observed Cases in ITT Population): Study 1
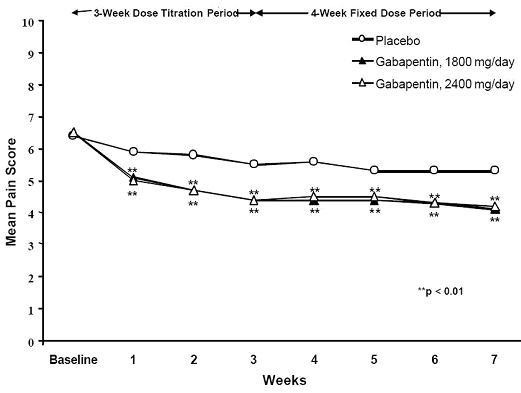
Figure 2. Weekly Mean Pain Scores (Observed Cases in ITT Population): Study 2
The proportion of responders (those patients reporting at least 50% improvement in endpoint pain score compared with baseline) was calculated for each study (Figure 3).
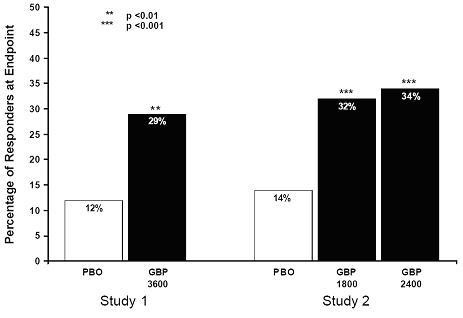
Figure 3. Proportion of Responders (patients withreduction in pain score) at Endpoint: Controlled PHN Studies
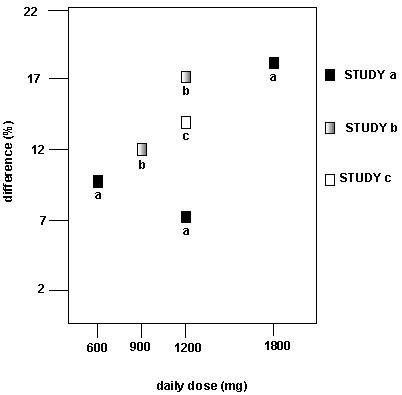
Figure 4. Responder Rate in Patients Receiving Gabapentin Expressed as a Difference from Placebo by Dose and Study: Adjunctive Therapy Studies in PatientsYears of Age with Partial Seizures
In the figure, treatment effect magnitude, measured on the Y axis in terms of the difference in the proportion of gabapentin and placebo assigned patients attaining a 50% or greater reduction in seizure frequency from baseline, is plotted against the daily dose of gabapentin administered (X axis).
Although no formal analysis by gender has been performed, estimates of response (Response Ratio) derived from clinical trials (398 men, 307 women) indicate no important gender differences exist. There was no consistent pattern indicating that age had any effect on the response to gabapentin. There were insufficient numbers of patients of races other than Caucasian to permit a comparison of efficacy among racial groups.
A fourth study in pediatric patients age 3 to 12 years compared 25 to 35 mg/kg/day gabapentin (N=118) with placebo (N=127). For all partial seizures in the intent-to-treat population, the response ratio was statistically significantly better for the gabapentin group (-0.146) than for the placebo group (-0.079). For the same population, the responder rate for gabapentin (21%) was not significantly different from placebo (18%).
A study in pediatric patients age 1 month to 3 years compared 40 mg/kg/day gabapentin (N=38) with placebo (N=38) in patients who were receiving at least one marketed antiepileptic drug and had at least one partial seizure during the screening period (within 2 weeks prior to baseline). Patients had up to 48 hours of baseline and up to 72 hours of double-blind video EEG monitoring to record and count the occurrence of seizures. There were no statistically significant differences between treatments in either the response ratio or responder rate.
INDICATIONS & USAGE
Postherpetic NeuralgiaEpilepsy
GABAPENTIN CONTRAINDICATIONS
WARNINGS
Suicidal Behavior and IdeationTABLE 2. Risk by indication for antiepileptic drugs in the pooled analysis
Indication Placebo
Patients with
Events Per 1000
Patients Drug Patients
with Events
Per 1000
Patients Relative Risk:
Incidence of
Events in Drug
Patients/Incidence
in Placebo
Patients Risk Difference:
Additional
Drug Patients
with Events
Per
1000 Patients
Neuropsychiatric Adverse EventsPatients 3 to 12 years of age
Withdrawal Precipitated Seizure, Status Epilepticus
Tumorigenic Potential
PRECAUTIONS: Carcinogenesis, Mutagenesis, Impairment of Fertility.) The clinical significance of this finding is unknown. Clinical experience during gabapentinpremarketing development provides no direct means to assess its potential for inducing tumors in humans.
In clinical studies in adjunctive therapy in epilepsy comprising 2085 patient-years of exposure in patients >12 years of age, new tumors were reported in 10 patients (2 breast, 3 brain, 2 lung, 1 adrenal, 1 non-Hodgkinlymphoma, 1 endometrial carcinoma in situ), and preexisting tumors worsened in 11 patients (9 brain, 1 breast, 1 prostate) during or up to 2 years following discontinuation of gabapentin. Without knowledge of the background incidence and recurrence in a similar population not treated with gabapentin, it is impossible to know whether the incidence seen in this cohort is or is not affected by treatment.
Sudden and Unexplained Death in Patients With Epilepsy
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as Multiorgan hypersensitivity, has been reported in patients taking antiepileptic drugs, including gabapentin. Some of these events have been fatal or life-threatening. DRESS typically, although not exclusively, presents with fever, rash, and/or lymphadenopathy, in association with other organ system involvement, such as hepatitis, nephritis, hematological abnormalities, myocarditis, or myositis sometimes resembling an acute viral infection. Eosinophilia is often present. Because this disorder is variable in its expression, other organ systems not noted here may be involved.
It is important to note that early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident. If such signs or symptoms are present, the patient should be evaluated immediately. Gabapentin should be discontinued if an alternative etiology for the signs or symptoms cannot be established.
PRECAUTIONS
Information for PatientsDrug Interactions).
Patients should be encouraged to enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry if they become pregnant. This registry is collecting information about the safety of antiepileptic drugs during pregnancy. To enroll, patients can call the toll free number 1-888-233-2334 (seePRECAUTIONS, Pregnancysection).
Prior to initiation of treatment with gabapentin, the patient should be instructed that a rash or other signs or symptoms of hypersensitivity (such as fever or lymphadenopathy) may herald a serious medical event and that the patient should report any such occurrence to a physician immediately.
Laboratory Tests
Drug Interactions
In vitro studies were conducted to investigate the potential of gabapentin to inhibit the major cytochrome P450 enzymes (CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4) that mediate drug and xenobiotic metabolism using isoform selective marker substrates and human liver microsomal preparations. Only at the highest concentration tested (171 mcg/mL; 1 mM) was a slight degree of inhibition (14% to 30%) of isoform CYP2A6 observed. No inhibition of any of the other isoforms tested was observed at gabapentin concentrations up to 171 mcg/mL (approximately 15 times the Cmax at 3600 mg/day).
PRECAUTIONS). Morphine pharmacokinetic parameter values were not affected by administration of gabapentin 2 hours after morphine. The magnitude of interaction at other doses is not known.
Cimetidine
In the presence of cimetidine at 300 mg QID (N=12) the mean apparent oral clearance of gabapentin fell by 14% and creatinine clearance fell by 10%. Thus cimetidine appeared to alter the renal excretion of both gabapentin and creatinine, an endogenous marker of renal function. This small decrease in excretion of gabapentin by cimetidine is not expected to be of clinical importance. The effect of gabapentin on cimetidine was not evaluated.
Oral Contraceptive
Based on AUC and half-life, multiple-dose pharmacokinetic profiles of norethindrone and thinyl estradiol following administration of tablets containing 2.5 mg of norethindrone acetate and 50 mcg of ethinyl estradiol were similar with and without coadministration of gabapentin (400 mg TID; N=13). The Cmax of norethindrone was 13%higher when it was coadministered with gabapentin; this interaction is not expected to be of clinical importance.
Antacid (Maalox
Maalox reduced the bioavailability of gabapentin (N=16) by about 20%. This decrease in bioavailability was about 5% when gabapentin was administered 2 hours after Maalox. It is recommended that gabapentin be taken at least 2 hours following Maalox administration.
Effect of Probenecid
Probenecid is a blocker of renal tubular secretion. Gabapentin pharmacokinetic parameters without and with probenecid were comparable. This indicates that gabapentin does not undergo renal tubular secretion by the pathway that is blocked by probenecid.
Drug/Laboratory Tests Interactions
Carcinogenesis, Mutagenesis, Impairment of Fertility
Pregnancy
Nursing Mothers
Pediatric Use
CLINICAL PHARMACOLOGY, Clinical Studies).
Geriatric Use
CLINICAL PHARMACOLOGY,ADVERSE REACTIONS, andDOSAGE AND ADMINISTRATIONsections).
GABAPENTIN ADVERSE REACTIONS
Postherpetic NeuralgiaIncidence in Controlled Clinical Trials
TABLE 3. Treatment-Emergent Adverse Event Incidence in Controlled Trials in Postherpetic Neuralgia (Events in at least 1% of Gabapentin-Treated Patients and Numerically More Frequent Than in the Placebo Group)
Body System/Preferred Term
Gabapentin
N=336
%
Placebo
N=227
%
Body as a Whole
Digestive System
Metabolic and Nutritional Disorders
Nervous System
Respiratory System
Skin and Appendages
Special Senses
Epilepsy
WARNINGS, Neuropsychiatric Adverse Events).
Approximately 7% of the 2074 patients >12 years of age and approximately 7% of the 449 pediatric patients 3 to 12 years of age who received gabapentin in premarketing clinical trials discontinued treatment because of an adverse event. The adverse events most commonly associated with withdrawal in patients >12 years of age were somnolence (1.2%), ataxia (0.8%), fatigue (0.6%), nausea and/or vomiting (0.6%), and dizziness (0.6%). The adverse events most commonly associated with withdrawal in pediatric patients were emotional lability (1.6%), hostility (1.3%), and hyperkinesia (1.1%).
Incidence in Controlled Clinical Trials
TABLE 4. Treatment-Emergent Adverse Event Incidence in Controlled Add-On Trials In Patients >12 years of age (Events in at least 1% of Gabapentin patients and numerically more frequent than in the placebo group)
Body System/Adverse Event Gabapentina
N=543
% Placeboa
N=378
%
Body As A Whole
Digestive System
Hematologic and Lymphatic Systems
Musculoskeletal System
Nervous System
Respiratory System
Skin and Appendages
Urogenital System
Special Senses
Laboratory Deviations
TABLE 5. Treatment-Emergent Adverse Event Incidence in Pediatric Patients Age 3 to 12 Years in a Controlled Add-On Trial (Events in at least 2% of Gabapentin patients and numerically more frequent than in the placebo group)
Body System/Adverse Event Gabapentina
N=119
% Placeboa
N=128
%
Body As A Whole
Digestive System
Nervous System
Respiratory System
Other Adverse Events Observed During All Clinical Trials
Body As A Whole:Frequent: asthenia, malaise, face edema; Infrequent: allergu, generalized edema, weight decrease, chill; Rare: strange feelings, lassitude, alcohol intolerance, hangover effect.
Cardiovascular System:Frequent: hypertension; Infrequent: hypotension, angina pectoris, peripheral vascular disorder, palpitation, tachycardia, migraine, murmur; Rare: atrial fibrillation, heart failure, thrombophlebitis, deep thrombophlebitis, myocardial infarction, cerebrovascular accident, pulmonary thrombosis, ventricular extrasystoles, bradycardia, premature atrial contraction, pericardial rub, heart block, pulmonary embolus, hyperlipidemia, hypercholesterolemia, pericardial effusion, pericardits.
Digestive System:salivary gland enlarged, lip hemorrhage, esophagitis, hiatal hernia, hematemesis, proctitis, irritable bowel syndrome, rectal hemorrhage, esophageal spasm.
Endocrine System:Rare: hyperthyroid, hypothyroid, goiter, hypoestrogen, ovarian failure, epididymitis, swollen testicle, cushingoid appearance.
Hematologic and Lymphatic System:lymphoma, bleeding time increased.
Musculoskeletal System:Frequent: arthralgia; Infrequent: tendinitis, arthritis, joint stiffness, joint swelling, positive Romberg test; Rare: costochondritis, osteoporosis, bursitis, contracture.
Nervous System:Frequent: vertigo, hyperkinesia, paresthesia, decreased or absent reflexes, increased reflexes, anxiety, hostility; Infrequent; CNS tumors, syncope, dreaming abnormal, aphasia, hypesthesia, intracranial hemorrhage, hypotonia, dysesthesia, paresis, dystonia, hemiplegia, facial paralysis, stupor, cerebellar dysfunction, positive Babinski sign, decreased position sense, subdural hematoma, apathy, hallucination, decrease or loss of libido, agitation, paranoia, depersonalization, euphoria, feeling high, doped-up sensation, psychosis; Rare: choreoathetosis, orofacial dyskinesia, encephalopathy, nerve palsy, personality disorder, increased libido, subdued temperament, apraxia, fine motor control disorder, meningismus, local myoclonus, hyperesthesia, hypokinesia, mania, neurosis, hysteria, antisocial reaction.
Respiratory System:Frequent: pneumonia; Infrequent: epistaxis, dyspnea, apnea; Rare: mucositis, aspiration pneumonia, hyperventilation, hiccup, laryngitis, nasal obstruction, snoring, bronchospasm, hypoventilation, lung edema.
Dermatological:Infrequent: alopecia, eczema, dry skin, increased sweating, urticaria, hirsutism, seborrhea, cyst, herpes simplex; Rare: herpes zoster, skin discolor, skin papules, photosensitive reaction, leg ulcer, scalp seborrhea, psoriasis, desquamation, maceration, skin nodules, subcutaneous nodule, melanosis, skin necrosis, loacl swelling.
Urogenital System:Infrequent: hematuria, dysuria, urination frequency, cystitis, urinary retention, urinary incontinence, vaginal hemorrhage, amenorrhea, dysmenorrhea, menorrhagia, breast cancer, unable to climax, ejaculation abnormal; Rare: kidney pain, leukorrhea, pruritus genital, renal stone, acute renal failure, anuria, glycosuria, nephrosis, nocturia, pyuria, urination urgency, vaginal pain, breast pain, testicle pain.
Special Senses:Frequent: abnormal vision; Infrequent: cataract, conjunctivitis, eyes dry, eye pain, visual field defect, photophobia, bilateral or unilateral ptosis, eye hemorrhage, hordeolum, hearing loss, earache, tinnitus, inner ear infection, otitis, taste loss, unusual taste, eye twitching, ear fullness; Rare: eye itching, abnormal accommodation, perforated ear drum, sensitivity to noise, eye focusing problem, watery eyes, retinopathy, glaucoma, iritis, corneal disorders, lacrimal dysfunction, degenerative eye changes, blindness, retinal degeneration, miosis, chorioretinitis, strabismus, eustachian tube dysfunction, labryinthitis, otitis externa, odd smell.
Body as a Whole:dehydration, infectious mononucleosis
Digestive System:hepatitis
Hemic and Lymphatic System:coagulation defect
Nervous System:aura disappeared, occipital neuralgia
Psychobiologic Function:sleepwalking
Respiratory System:pseudocroup, hoarseness
Body as a Whole:Infrequent: chest pain, cellulitis, malaise, neck pain, face edema, allergic reaction, abscess, chills, chills and fever, mucous membrane disorder; Rare: body odor, cyst, fever, hernia, abnormal BUN value, lump in neck, pelvic pain, sepsis, viral infection.
Cardiovascular System:Infrequent: hypertension, syncope, palpitation, migraine, hypotension, peripheral vascular disorder, cardiovascular disorder, cerebrovascular accident, congestive heart failure, myocardial infarction, vasodilatation; rare: angina pectoris, heart failure, increased capillary fragility, phlebitis, thrombophlebitis, varicose vein.
Digestive System:Infrequent: gastroenteritis, increased appetite, gastrointestinal disorder, oral moniliasis, gastritis, tongue disorder, thirst, tooth disorder, abnormal stools, anorexia, liver function tests abnormal, periodontal abscess; Rare: cholelithiasis, duodenal ulcer, fecal incontinence, gamma glutamyl transpeptidase increased, gingivitis, intestinal obstruction, intestinal ulcer, melena, mouth ulceration, rectal disorder, rectal hemorrhage, stomatitis.
Endocrine System:Infrequent: diabetes mellitus.
Hemic and Lymphatic System:Infrequent: ecchymosis, anemia; Rare: lymphadenopathy, lymphoma-like reaction, prothrombin decreased.
Metabolic and Nutritional:Infrequent: edema, gout, hypoglycemia, weight loss; rare: alkaline phosphatase, increased, diabetic ketoacidosis, lactic dehydrogenase increased.
Musculoskeletal:Infrequent: arthritis, arthralgia, myalgia, arthrosis, leg cramps, myasthenia; Rare: shin bone pain, joint disorder, tendon disorder.
Nervous System:Frequent: confusion, depression; Infrequent: vertigo, nervousness, paresthesi, insomnia, neuropathy, libido decreased, anxiety, depersonalization, reflexes decreased, speech disorder, abnormal dreams, dysarthria, emotional lability, nystagmus, stupor, circumoral paresthesia, euphoria, hyperesthesia, hypokinesia; Rare: agitation, hypertonia, libido increased, movement disorder, myoclonus, vestibular disorder.
Respiratory System:Infrequent: cough increased, bronchitis, rhinitis, sinusitis, pneumonia, asthma, lung disorder, epistaxis; Rare: hemoptysis, voice alteration.
Skin and Appendages:Infrequent: pruritus, skin ulcer, dry skin, herpes zoster, skin disorder, fungal dermatitis, furunculosis, herpes simplex, psoriasis, sweating, urticaria, vesiculobullous rash; Rare: acne, hair disorder, maculopapular rash, nail disorder, skin carcinoma, skin discoloration, skin hypertrophy.
Special Senses:Infrequent: abnormal vision, ear pain, eye disorder, taste perversion, deafness; Rare: conjunctival hyperemia, diabetic retinopathy, eye pain, fundi with microhemorrhage, retinal vein thrombosis, tast loss.
Urogenital System:Infrequent: urinary tract infection, dysuria, impotence, urinary incontinence, vaginal moniliasis, breast pain, menstrual disorder, polyuria, urinary retention; Rare: cystitis, ejaculation abnormal, swollen penis, gynecomastia, nocturia, pyelonephritis, swollen scrotum, urinary frequency, urinary urgency, urine abnormality.
Postmarketing and Other Experience
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
DOSAGE & ADMINISTRATION
Postherpetic Neuralgia
Epilepsy
CLINICAL PHARMACOLOGY, Pediatrics.) Dosages up to 50 mg/kg/day have been well-tolerated in a long-term clinical study. The maximum time interval between doses should not exceed 12 hours.
It is not necessary to monitor gabapentin plasma concentrations to optimize gabapentin therapy. Further, because there are no significant pharmacokinetic interactions among gabapentin and other commonly used antiepileptic drugs, the addition of gabapentin does not alter the plasma levels of these drugs appreciably.
If gabapentin is discontinued and/or an alternate anticonvulsant medication is added to the therapy, this should be done gradually over a minimum of 1 week.
Dosage in Renal Impairment
TABLE 6. Gabapentin Dosage Based on Renal Function
Renal Function
Creatinine Clearance
(mL/min) Total Daily
Dose Range
(mg/day) Dose Regimen (mg)
Post-Hemodialysis Supplemental Dose (mg)b
Dosage in Elderly
HOW SUPPLIED
Gabapentin Capsules USP, 100 mg are white/white size '3' hard gelatin capsules imprinted with 'D' on white cap andon white body with black edible ink filled with white to off-white crystalline powder.Bottles of 100 NDC 65862-198-01
Bottles of 500 NDC 65862-198-05
Bottles of 1000 NDC 65862-198-99
Gabapentin Capsules USP, 300 mg are yellow/yellow size'1' hard gelatin capsules imprinted with 'D' on yellow cap andon yellow body with black edible ink filled with white to off-white crystalline powder.
Bottles of 100 NDC 65862-199-01
Bottles of 500 NDC 65862-199-05
Bottles of 1000 NDC 65862-199-99
Gabapentin Capsules USP, 400 mg are orange/orange size'0' hard gelatin capsules imprinted with'D' on orange cap andon orange body with black edible ink filled with white to off-white crystalline powder.
Bottles of 100 NDC 65862-200-01
Bottles of 500 NDC 65862-200-05
STORAGE AND HANDLING
Store at 20 to 25 C (68 to 77 F) excursions permitted to 15 to 30 C(59 to 86 F)[See USP Controlled Room Temperature].MEDICATION GUIDE
Gabapentin Capsules, USPRx only
Read the Medication Guide before you start taking gabapentin capsules and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
What is the most important information I should know about gabapentin capsules?
Do not stop taking gabapentin capsules without first talking to your healthcare provider.Stopping gabapentin capsules suddenly can cause serious problems.
Gabapentin capsules can cause serious side effects including:
1. Like other antiepileptic drugs, gabapentin capsules may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
How can I watch for early symptoms of suicidal thoughts and actions?
Do not stop taking gabapentin capsules without first talking to a healthcare provider.
2. Changes in behavior and thinking- Using gabapentin capsules in children 3 to 12 years of age can cause emotional changes, aggressive behavior, problems with concentration, restlessness, changes in school performance, and hyperactivity.
3. Gabapentin capsules may cause a serious or life-threatening allergic reactionthat may affect your skin or other parts of your body such as your liver or blood cells. You may or may not have rash when you get this type of reaction. It may cause you to be hospitalized or to stop gabapentin capsules. Call a healthcare provider right away if you have any of the following symptoms:
What are gabapentin capsules?
Gabapentin capsules are a prescription medicine used to treat:
Who should not take gabapentin capsules?
Do not take gabapentin capsules if you are allergic to gabapentin or any of the other ingredients in gabapentin capsules. See the end of this Medication Guide for a complete list of ingredients in gabapentin capsules.
What should I tell my healthcare provider before taking gabapentin capsules?
Before taking gabapentin capsules, tell your healthcare provider if you:
Tell your healthcare provider about all the medicines you take,including prescription and non-prescription medicines, vitamins, and herbal supplements.
Taking gabapentin capsules with certain other medicines can cause side effects or affect how well they work. Do not start or stop other medicines without talking to your healthcare provider.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take gabapentin capsules?
What should I avoid while taking gabapentin capsules?
What are the possible side effects of gabapentin capsules?
is the most important information I should know about gabapentin capsules?
The most common side effects of gabapentin capsules include:
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store gabapentin capsules?
Keep gabapentin capsules and all medicines out of the reach of children.
General information about the safe and effective use of gabapentin capsules
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use gabapentin capsules for a condition for which it was not prescribed. Do not give gabapentin capsules to other people, even if they have the same symptoms that you have. They may harm them.
This Medication Guide summarizes the most important information about gabapentin capsules. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about gabapentin capsules that was written for healthcare professionals.
For more information about gabapentin capsules and for medical inquiries or to report side effects regarding gabapentin capsules, please call 1-866-850-2876.
What are the ingredients in gabapentin capsules?
Active ingredient:gabapentin
Inactive ingredients:corn starch and talc. The empty hard gelatin capsule shell consists of gelatin, titanium dioxide, and sodium lauryl sulfate. In addition 300 mg also contains yellow iron oxide and 400 mg also contains yellow iron oxide and red iron oxide. The capsules are printed with edible ink containing black iron oxide and shellac.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

GabapentinGABAPENTIN CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!