Furosemide
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNINGS
- FUROSEMIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- FUROSEMIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- FUROSEMIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
WARNINGS
WARNINGFurosemide is a potent diuretic which, if given in excessive amounts, can lead to a profound diuresis with water and electrolyte depletion. Therefore, careful medical supervision is required and dose and dose schedule must be adjusted to the individual patient's needs. (See DOSAGE AND ADMINISTRATION.)
FUROSEMIDE DESCRIPTION
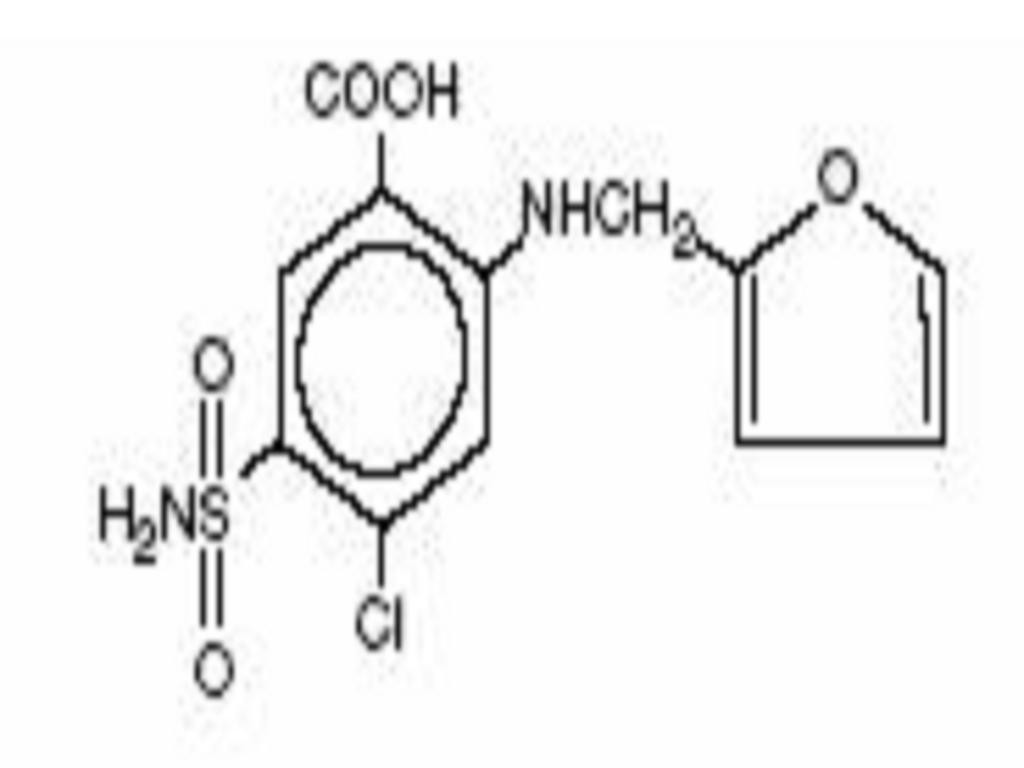
CLINICAL PHARMACOLOGY
Geriatric Population
PRECAUTIONS:Geriatric Use
INDICATIONS & USAGE
EdemaHypertension
FUROSEMIDE CONTRAINDICATIONS
WARNINGS
Drug Interactions
PRECAUTIONS
GeneralINFORMATION FOR PATIENTS
LABORATORY TESTS
Pediatric Use
DRUG INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
PRECAUTIONS: GeneralDOSAGE AND ADMINISTRATION
FUROSEMIDE ADVERSE REACTIONS
Gastrointestinal System Reactions
Systemic Hypersensitivity Reactions
Central Nervous System Reactions
Hematologic Reactions
Dermatologic-Hypersensitivity Reactions
Cardiovascular Reaction
Other Reactions
OVERDOSAGE
DOSAGE & ADMINISTRATION
Edema
PRECAUTIONS: Laboratory Tests.
PRECAUTIONS: Geriatric Use
Hypertension
PRECAUTIONS: Geriatric Use
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

FurosemideFurosemide TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!