Flomax
FULL PRESCRIBING INFORMATION: CONTENTS*
- FLOMAX DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- CLINICAL STUDIES
- INDICATIONS & USAGE
- FLOMAX CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- PREGNANCY
- GERIATRIC USE
- NURSING MOTHERS
- PEDIATRIC USE
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- FLOMAX ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL PATIENT PACKAGE INSERT
- INACTIVE INGREDIENT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
FLOMAX DESCRIPTION
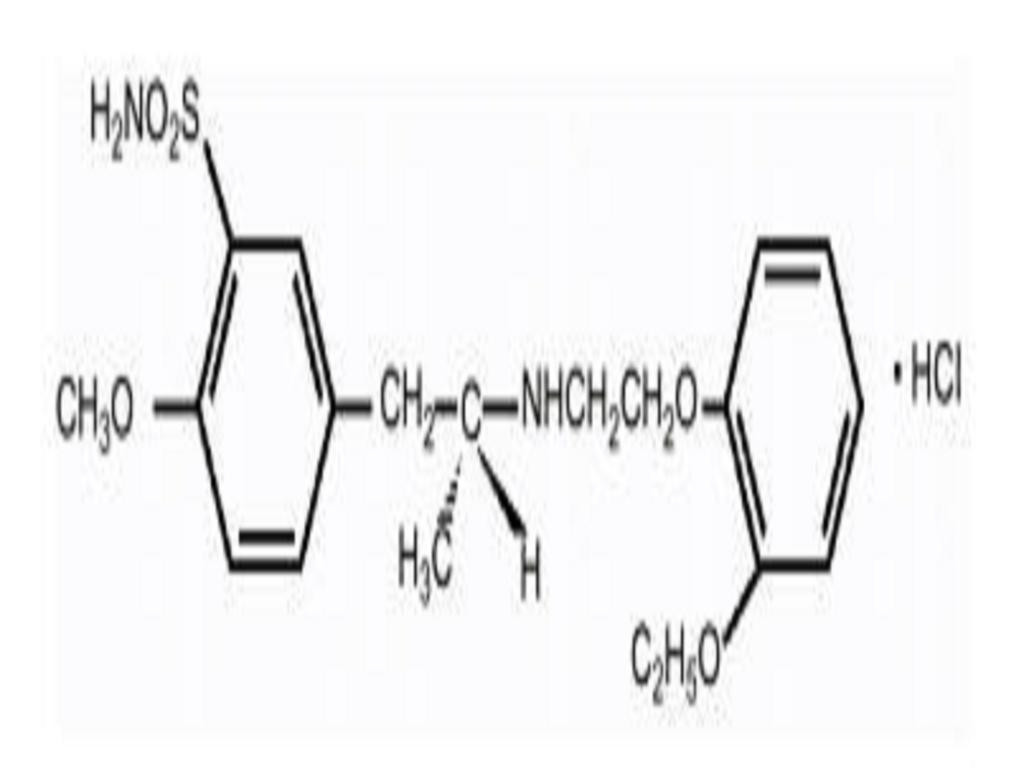
CLINICAL PHARMACOLOGY
PHARMACOKINETICS
PharmacokineticsAbsorption
Effect of Food
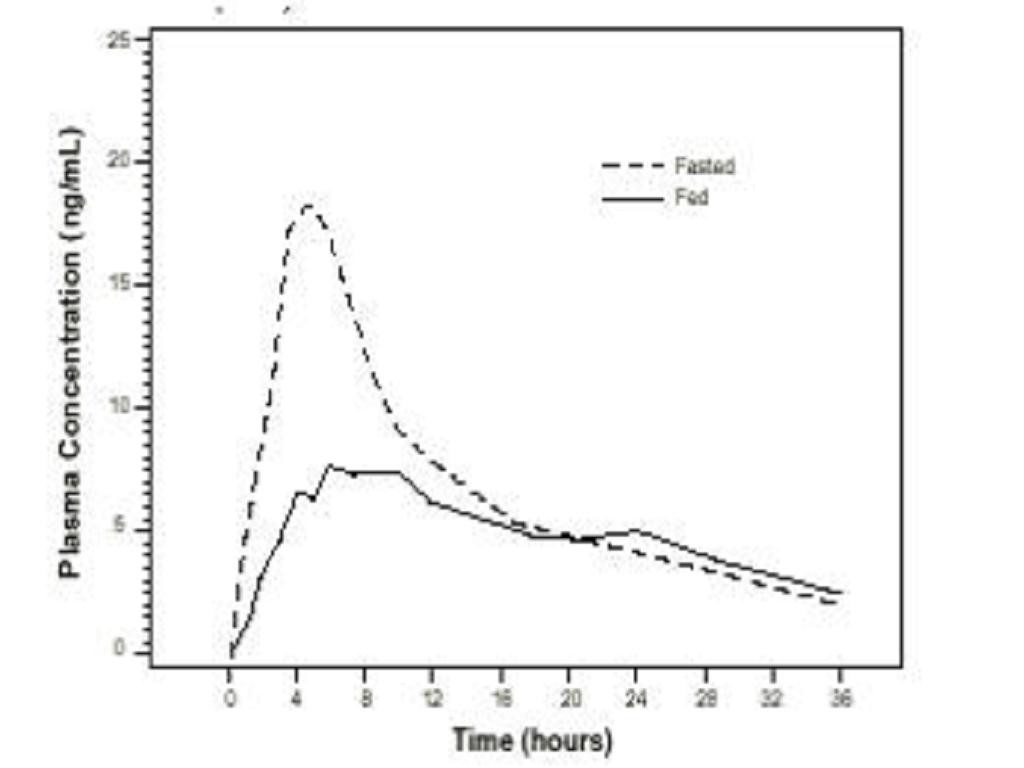
Pharmacokinetic0.4 mg QD to healthyParameter18-32 years)(age range 55 - 75 years)LightLightHigh-FatBreakfastFastedBreakfastBreakfastFasted
Distribution
Metabolism
Drug-Drug InteractionsCytochrome P450 Inhibition
Excretion
Special Populations
Geriatrics (Age)
Renal Dysfunction
Hepatic Dysfunction
Drug-Drug Interactions
Nifedipine, Atenolol, Enalapril
Warfarin
Digoxin and Theophylline
Furosemide
The pharmacokinetic and pharmacodynamic interaction between Flomax(tamsulosin hydrochloride) capsules 0.8 mg/day (steady-state) and furosemide 20 mg intravenously (single dose) was evaluated in ten healthy volunteers (age range 21-40 years). FLOMAX capsules had no effect on the pharmacodynamics (excretion of electrolytes) of furosemide. While furosemide produced an 11% to 12% reduction in tamsulosin hydrochloride Cmax and AUC, these changes are expected to be clinically insignificant and do not require adjustment of the FLOMAX capsules dosage.
Cytochrome P450 Inhibition:
Cimetidine
The effects of cimetidine at the highest recommended dose (400 mg every six hours for six days) on the pharmacokinetics of a single FLOMAX capsule 0.4 mg dose was investigated in ten healthy volunteers (age range 21-38 years). Treatment with cimetidine resulted in a significant decrease (26%) in the clearance of tamsulosin hydrochloride, which resulted in a moderate increase in tamsulosin hydrochloride AUC (44%). Therefore, FLOMAX capsules should be used with caution in combination with cimetidine, particularly at doses higher than 0.4 mg.
Strong Inhibitors of CYP3A4 or CYP2D6
The effects of ketoconazole (a strong inhibitor of CYP3A4) at 400 mg every day on the pharmacokinetics of a single FLOMAX capsule 0.4 mg dose was investigated in 24 healthy volunteers (age range from 23 to 47 years). Treatment with ketoconazole resulted in a Cmax and AUC that increased by a factor of 2.2 and 2.8, respectively. Therefore, FLOMAX 0.4 mg capsules should be used with caution in combination with strong inhibitors of CYP3A4. Doses above 0.4 mg should not be used in combination with strong inhibitors of CYP3A4 (e.g., ketoconazole).
The effects of paroxetine (a strong inhibitor of CYP2D6) at 20 mg every day on the pharmacokinetics of a single FLOMAX capsule 0.4 mg dose was investigated in 23 healthy volunteers (age range from 23 to 48 years). Treatment with paroxetine resulted in a Cmax and AUC that increased by a factor of 1.3 and 1.6, respectively. Therefore, FLOMAX capsules should be used with caution in combination with strong inhibitors of CYP2D6, particularly at doses higher than 0.4 mg (e.g., 0.8 mg).
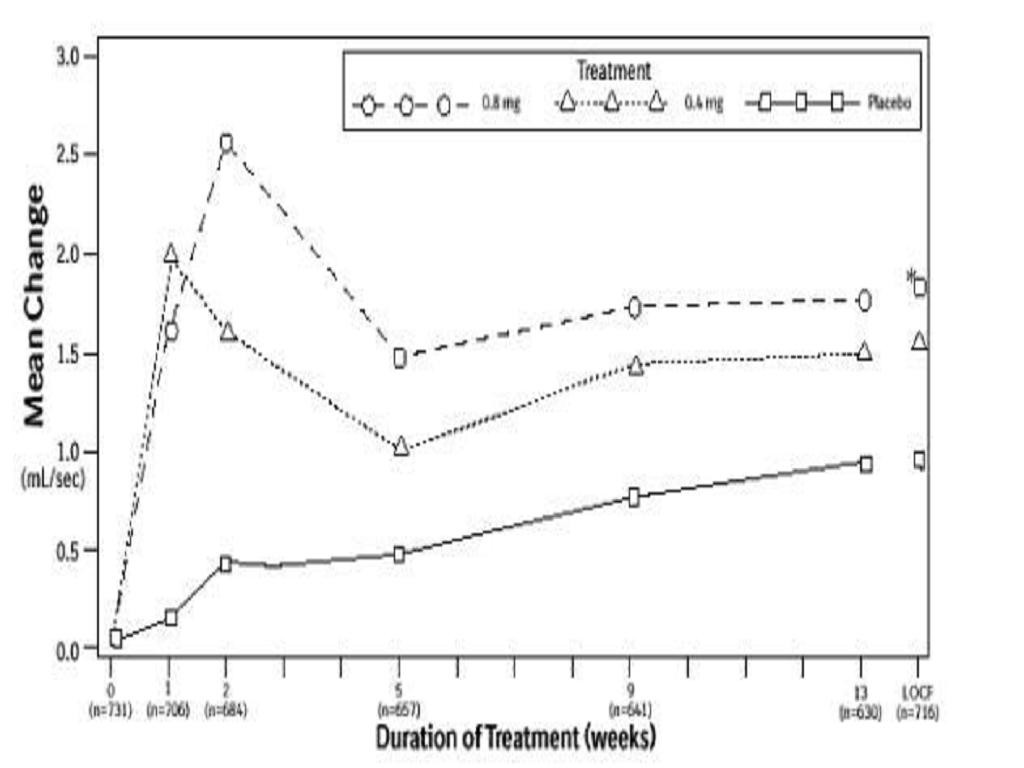
CLINICAL STUDIES
CLINICAL STUDIESTotal AUA Symptom ScorePeak Urine Flow RateMean BaselineMeanMean BaselineMeanValueChangeValueChangeStudy 1Study 2



INDICATIONS & USAGE
INDICATIONS AND USAGEFLOMAX CONTRAINDICATIONS
CONTRAINDICATIONSWARNINGS
ADVERSE REACTIONSPRECAUTIONS, Information for Patients
PRECAUTIONS
GeneralCLINICAL PHARMACOLOGYDrug-Drug Interactions
WARNINGS
Drug-Drug Interactions
INFORMATION FOR PATIENTS
PATIENT INFORMATIONLABORATORY TESTS
Laboratory TestsPREGNANCY
GERIATRIC USE
Geriatric UseCLINICAL PHARMACOLOGY, PharmacokineticsSpecial PopulationsGeriatrics (Age))
NURSING MOTHERS
Nursing MothersPEDIATRIC USE
Pediatric UseCARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Carcinogenesis, Mutagenesis, and Impairment of FertilityFLOMAX ADVERSE REACTIONS
ADVERSE REACTIONSBODY SYSTEM/FLOMAX CAPSULES GROUPSPLACEBOADVERSE EVENT0.4mg0.8mgn=502n=492n=493BODY AS WHOLENERVOUS SYSTEMRESPIRATORY SYSTEMDIGESTIVE SYSTEMUROGENITAL SYSTEMSPECIAL SENSES
-
● The adverse event occurred for the first time after initial dosing with double-blind study medication.
-
● The adverse event was present prior to or at the time of initial dosing with double-blind study medication and subsequently increased in severity during double-blind treatment; or
-
● The adverse event was present prior to or at the time of initial dosing with double-blind study medication, disappeared completely, and then reappeared during double-blind treatment.
WARNINGS
Post-Marketing Experience
PRECAUTIONS, General
OVERDOSAGE
OVERDOSAGEWARNINGSADVERSE REACTIONS
One patient reported an overdose of thirty 0.4 mg FLOMAX capsules. Following the ingestion of the capsules, the patient reported a severe headache.
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATIONWARNINGS
HOW SUPPLIED
STORAGE AND HANDLING
SPL PATIENT PACKAGE INSERT
-
● FLOMAX is not for women.
-
● FLOMAX is not for children.
-
● Before taking FLOMAX, tell your doctor about all your medical conditions including:
-
● any kidney or liver problems.
-
● any history of low blood pressure.
-
● any allergies to sulfa or any other medicines.
-
● if you are planning to have cataract surgery.
-
● Tell your doctor about all the medicines you take including:
-
● any prescription medicines, including blood pressure medicines
-
● any non-prescription medicines, including vitamins and herbal supplements.
-
● Some of your other medicines may affect the way FLOMAX works. Especially tell your doctor if you take a medicine for high blood pressure. You should not take FLOMAX if you are already taking certain blood pressure medicines.
-
● Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine.
-
● How should I take FLOMAX?
-
● Take FLOMAX exactly as prescribed by your doctor.
-
● Do not crush, chew, or open FLOMAX capsules.
-
● Take FLOMAX one time each day, about 30 minutes after the same meal each day. For example, you may take FLOMAX 30 minutes after dinner each day.
-
● If you miss a dose of FLOMAX, take it as soon as you remember. If you miss your dose for the whole day, continue with your next dose on your regular schedule. Do not take two doses at the same time.
-
● If you stop or forget to take FLOMAX for several days, talk with your doctor before starting again.
-
● If you take more FLOMAX capsules than prescribed, call your doctor right away.
-
● Decreased blood pressure when changing positions. FLOMAX capsules may cause a sudden drop in blood pressure upon standing, especially after the first dose or when changing doses. Symptoms may include:
-
● fainting
-
● dizziness
-
● lightheadedness
-
● Change positions slowly from lying down to sitting up or from a sitting to a standing position until you learn how you react to FLOMAX capsules. If you begin to feel dizzy, sit or lie down until you feel better. If the symptoms are severe or do not improve, call your doctor.
-
● Allergic reactions. Make your doctor aware of any allergic reactions you may experience while taking FLOMAX.
-
● rash
-
● itching
-
● hives
-
● swelling of face, tongue, or throat
-
● difficulty breathing
-
● Get medical help right away if you have swelling of the face, tongue or throat, or difficulty breathing.
-
● A painful erection that will not go away. FLOMAX capsules can cause a painful erection (priapism), which cannot be relieved by having sex. If this happens, get medical help right away. If priapism is not treated, you may not be able to get an erection in the future.
-
● Eye problems during cataract surgery. During cataract surgery, a condition called intraoperative floppy iris syndrome (IFIS) can happen if you take or have taken FLOMAX. If you need to have cataract surgery, be sure to tell your surgeon if you take or have taken FLOMAX.
-
● runny nose
-
● dizziness
-
● decreased semen
-
● Active Ingredient: tamsulosin hydrochloride
-
● Inactive Ingredients: methacrylic acid copolymer dispersion NF, microcrystalline cellulose, triacetin, calcium stearate, talc, FD&C blue No. 2, titanium dioxide, ferric oxide, gelatin, and trace amounts of black edible ink.
INACTIVE INGREDIENT
INACTIVE INGREDIENTS:CELLULOSE, MICROCRYSTALLINE
TRIACETIN
CALCIUM STEARATE
TALC
FD&C BLUE NO. 2
TITANIUM DIOXIDE
FERRIC OXIDE
GELATIN
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

FlomaxFlomax CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!