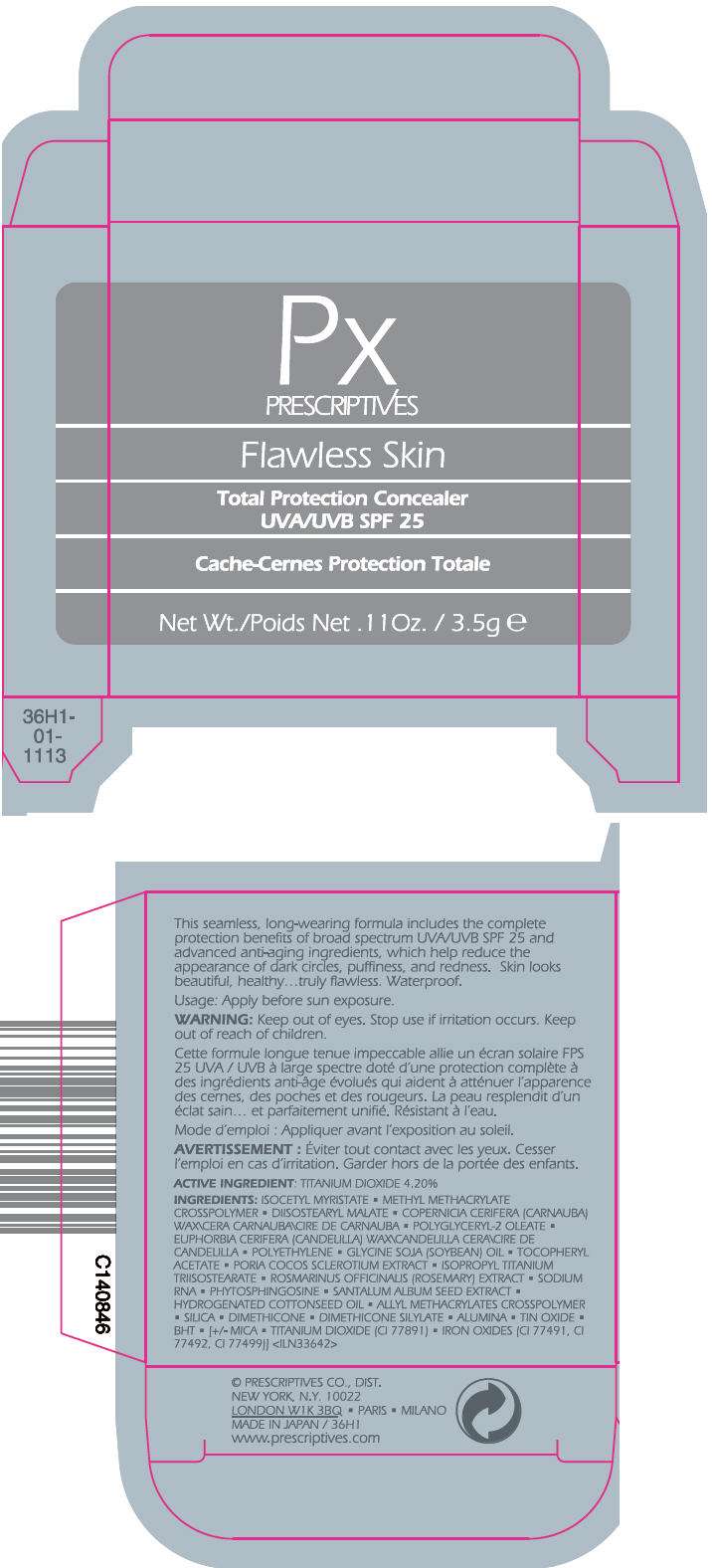
FLAWLESS SKIN TOTAL PROTECTION CONCEALER SPF 25
FLAWLESS SKIN TOTAL PROTECTION CONCEALER SPF 25
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Usage
Apply before sun exposure.
WARNING
Keep out of eyes.
Stop use if irritation occurs.
Keep out of reach of children.
ACTIVE INGREDIENT
TITANIUM DIOXIDE 4.20%
INGREDIENTS
ISOCETYL MYRISTATE • METHYL METHACRYLATE CROSSPOLYMER • DIISOSTEARYL MALATE • COPERNICIA CERIFERA (CARNAUBA) WAX\CERA CARNAUBA\CIRE DE CARNAUBA • POLYGLYCERYL-2 OLEATE • EUPHORBIA CERIFERA (CANDELILLA) WAX\CANDELILLA CERA\CIRE DE CANDELILLA • POLYETHYLENE • GLYCINE SOJA (SOYBEAN) OIL • TOCOPHERYL ACETATE • PORIA COCOS SCLEROTIUM EXTRACT • ISOPROPYL TITANIUM TRIISOSTEARATE • ROSMARINUS OFFICINALIS (ROSEMARY) EXTRACT • SODIUM RNA • PHYTOSPHINGOSINE • SANTALUM ALBUM SEED EXTRACT • HYDROGENATED COTTONSEED OIL • ALLYL METHACRYLATES CROSSPOLYMER • SILICA • DIMETHICONE • DIMETHICONE SILYLATE • ALUMINA • TIN OXIDE • BHT • [+/- MICA • TITANIUM DIOXIDE (CI 77891) • IRON OXIDES (CI 77491, CI 77492, CI 77499)]
PRESCRIPTIVES CO., DIST.
NEW YORK, N.Y. 10022
PRINCIPAL DISPLAY PANEL - 3.5g Container Carton
PX
PRESCRIPTIVES
Flawless Skin
Total Protection Concealer
UVA/UVB SPF 25
Net Wt./Poids Net . 11 Oz. / 3.5g e
FLAWLESS SKIN TOTAL PROTECTION CONCEALER SPF 25TITANIUM DIOXIDE PASTE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||