Exuviance Multi Protective Day
Exuviance Multi Protective Day Crème SPF 20
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredients
- Purpose
- Exuviance Multi Protective Day Uses
- Warnings
- Directions
- Inactive ingredients
- Exuviance Multi Protective Day Other information
- Questions or comments?
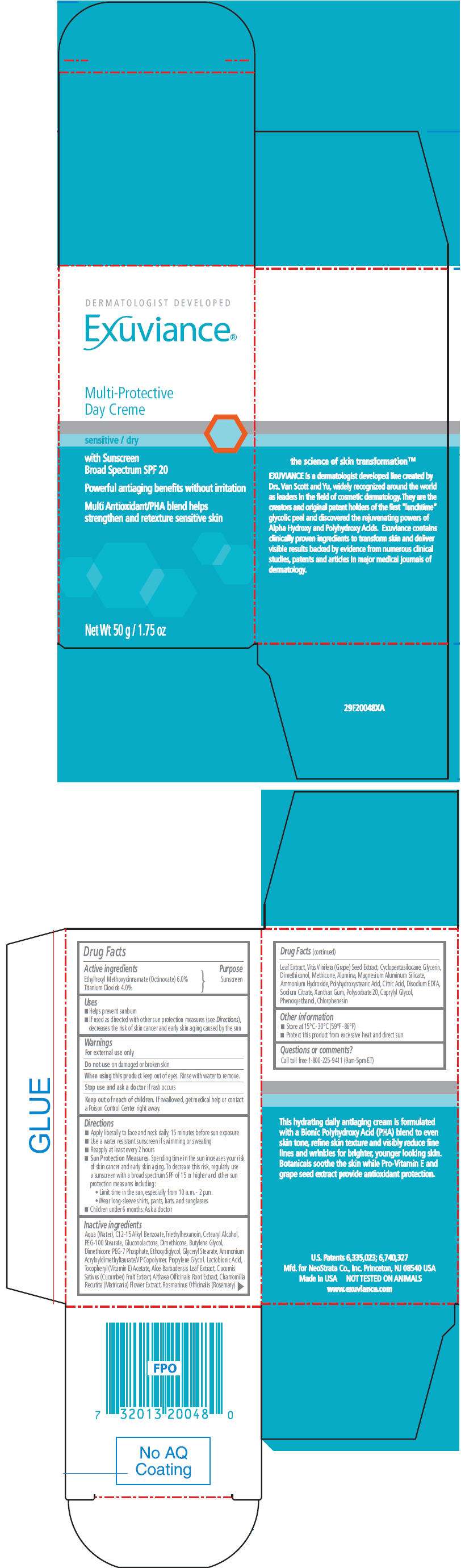
- PRINCIPAL DISPLAY PANEL - 50 g Bottle Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredients
Ethylhexyl Methoxycinnamate (Octinoxate) 6.0%
Titanium Dioxide 4.0%
Purpose
Sunscreen
Exuviance Multi Protective Day Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions ), decreases the risk of skin cancer and early skin aging caused by the sun
Warnings
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply liberally to face and neck daily, 15 minutes before sun exposure
- Use a water resistant sunscreen if swimming or sweating
- Reapply at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeve shirts, pants, hats, and sunglasses
- Children under 6 months: Ask a doctor
Inactive ingredients
Aqua (Water), C12-15 Alkyl Benzoate, Triethylhexanoin, Cetearyl Alcohol, PEG-100 Stearate, Gluconolactone, Dimethicone, Butylene Glycol, Dimethicone PEG-7 Phosphate, Ethoxydiglycol, Glyceryl Stearate, Ammonium Acryloyldimethyltaurate/VP Copolymer, Propylene Glycol, Lactobionic Acid, Tocopheryl (Vitamin E) Acetate, Aloe Barbadensis Leaf Extract, Cucumis Sativus (Cucumber) Fruit Extract, Althaea Officinalis Root Extract, Chamomilla Recutita (Matricaria) Flower Extract, Rosmarinus Officinalis (Rosemary) Leaf Extract, Vitis Vinifera (Grape) Seed Extract, Cyclopentasiloxane, Glycerin, Dimethiconol, Methicone, Alumina, Magnesium Aluminum Silicate, Ammonium Hydroxide, Polyhydroxystearic Acid, Citric Acid, Disodium EDTA, Sodium Citrate, Xanthan Gum, Polysorbate 20, Caprylyl Glycol, Phenoxyethanol, Chlorphenesin
Exuviance Multi Protective Day Other information
- Store at 15°C-30°C (59°F-86°F)
- Protect this product from excessive heat and direct sun
Questions or comments?
Call toll free 1-800-225-9411 (9am-5pm ET)
PRINCIPAL DISPLAY PANEL - 50 g Bottle Carton
DERMATOLOGIST DEVELOPED
Exuviance®
Multi-Protective
Day Creme
sensitive / dry
with Sunscreen
Broad Spectrum SPF 20
Powerful antiaging benefits without irritation
Multi Antioxidant/PHA blend helps
strengthen and retexture sensitive skin
Net Wt 50 g / 1.75 oz