EXSEL ALOE
ARMY AND AIR FORCE EXCHANGE SERVICE
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- EXSEL ALOE Uses
- Warnings
- Directions
- Inactive ingredients
- Principal Display Panel
- Questions or comments?
FULL PRESCRIBING INFORMATION
Active ingredient
Lidocaine HCl 0.5%
Purpose
Topical Analgesic
EXSEL ALOE Uses
- temporary relief of pain and itching
- helps relieve and soothes pain from sunburn, minor burns, cuts, scrapes, skin irritations and insect bites
Warnings
For external use only
Do not use
in large quantities, particularly over raw surfaces or blistered areas.
When using this product
- avoid contact with eyes
Stop use and ask a doctor if
- condition worsens or if symptoms persist for more than 7 days.
- symptoms clear up and occur again within a few days.
Keep out of reach of children
If swallowed get medical help or contact a Poison Control Center right away
Directions
- adults and children 2 years of age and older: apply to affected areas not more than 3 to 4 times daily
- children under 2 years of age: do not use, ask a doctor
Inactive ingredients
Water, Propylene Glycol, Glycerin, Isopropyl Alcohol, Triethanolamine, Polysorbate 80, Carbomer, Aloe Barbadensis Leaf Juice Powder, Menthol, Disodium EDTA, Diazolidinyl Urea, Yellow 5, Blue 1.
Principal Display Panel
exchange select
X
BURN RELIEF
Pain Relieving Gel
with
Lidocaine
Cools, Soothes and Relieves Sunburned Skin
NET WT. 8 OZ. (226 g)
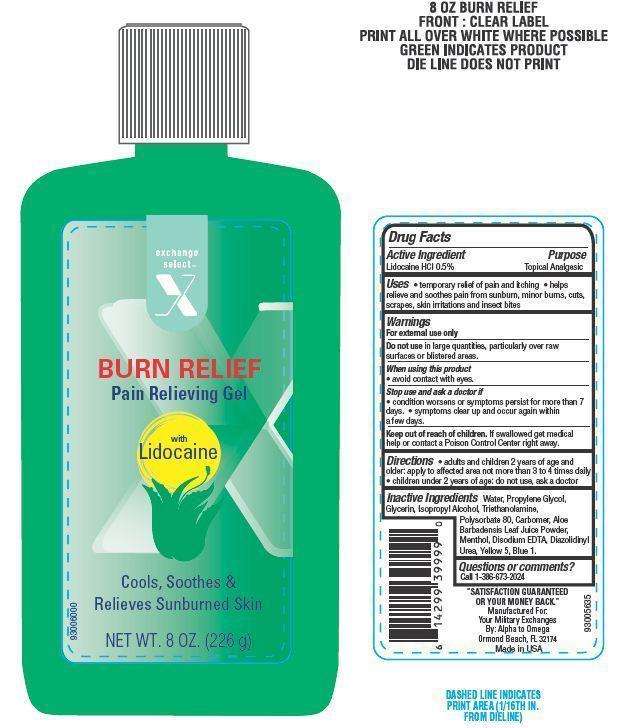
Questions or comments?
Call 1-386-673-2024
EXSEL ALOELIDOCAINE HYDROCHLORIDE GEL
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!