Ethambutol Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- ETHAMBUTOL HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- ANIMAL PHARMACOLOGY & OR TOXICOLOGY
- INDICATIONS & USAGE
- ETHAMBUTOL HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DRUG INTERACTIONS
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- ETHAMBUTOL HYDROCHLORIDE ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
ETHAMBUTOL HYDROCHLORIDE DESCRIPTION
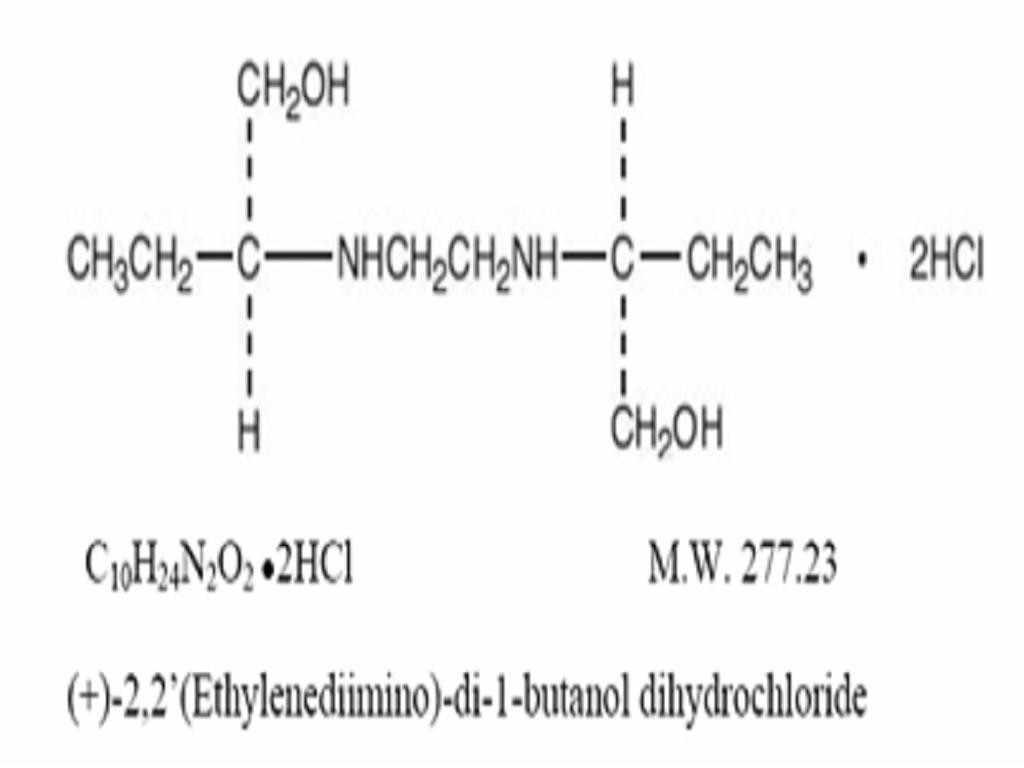
CLINICAL PHARMACOLOGY
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
INDICATIONS & USAGE
-
● Ethambutol Hydrochloride Tablets plus isoniazid
-
● Ethambutol Hydrochloride Tablets plus isoniazid plus streptomycin.
ETHAMBUTOL HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
PRECAUTIONSADVERSE REACTIONSADVERSE REACTIONS
PRECAUTIONS
ADVERSE REACTIONS
DRUG INTERACTIONS
PREGNANCY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
ETHAMBUTOL HYDROCHLORIDE ADVERSE REACTIONS
WARNINGS
www.fda.gov/medwatch <%0A%09%09%09%09%09%09%09>
DOSAGE & ADMINISTRATION
Initial Treatment:
Retreatment:
HOW SUPPLIED
-
● Bottles of 100 tablets.
-
● Bottles of 60 tablets.
-
● Bottles of 90 tablets.
-
● Bottles of 100 tablets.
-
● Bottles of 1000 tablets.
-
● Unit Dose Boxes of 100 tablets.
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Ethambutol HydrochlorideEthambutol Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!