ESIKA Perfect Match
ESIKA Perfect Match Lipstick SPF 20 + Liquid Lipliner
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Purpose
- ESIKA Perfect Match Uses
- Warnings
- Directions
- ESIKA Perfect Match Other information
- Inactive Ingredients
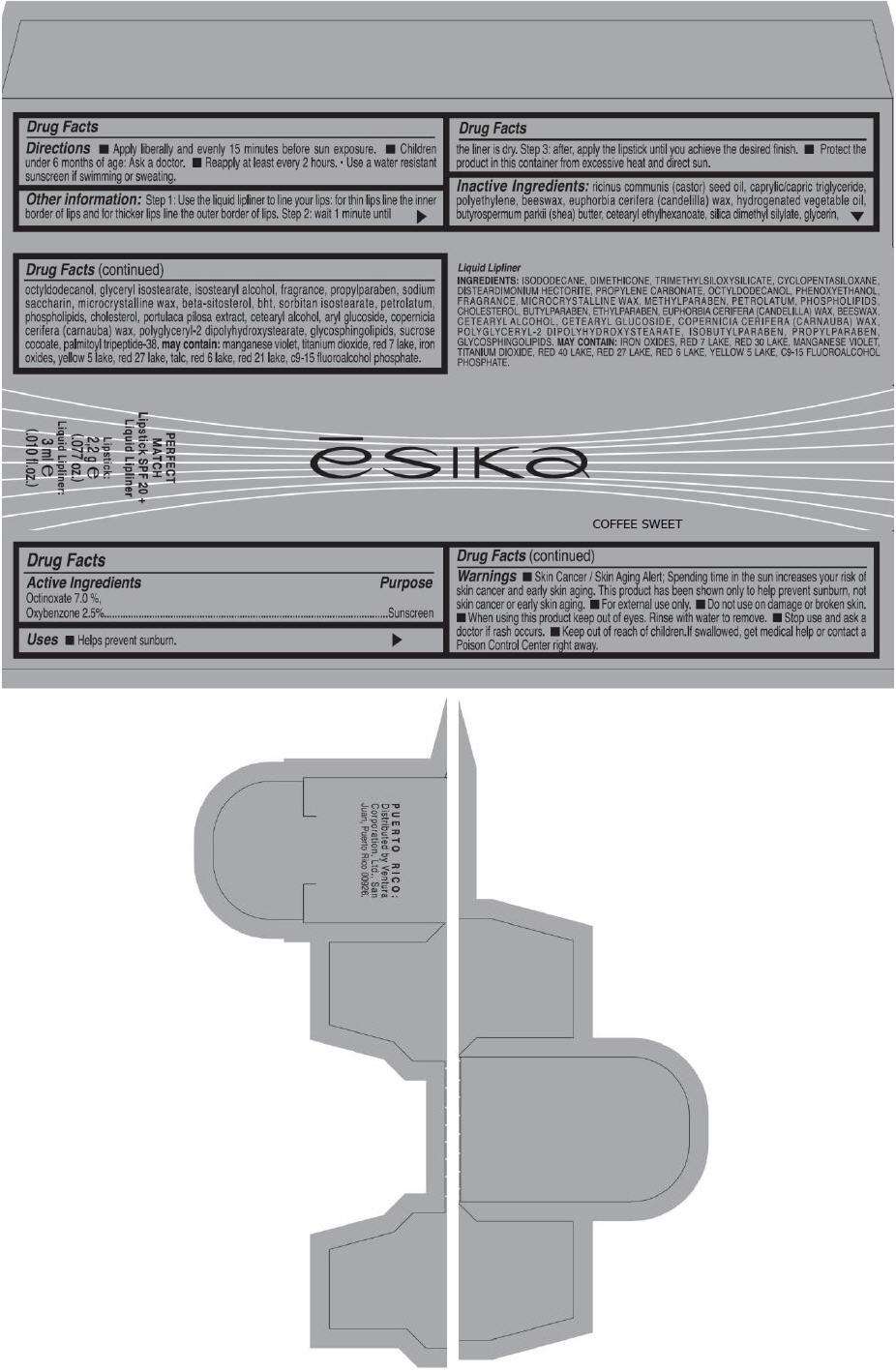
- PRINCIPAL DISPLAY PANEL - 50 g Tube Carton - COFFEE SWEET
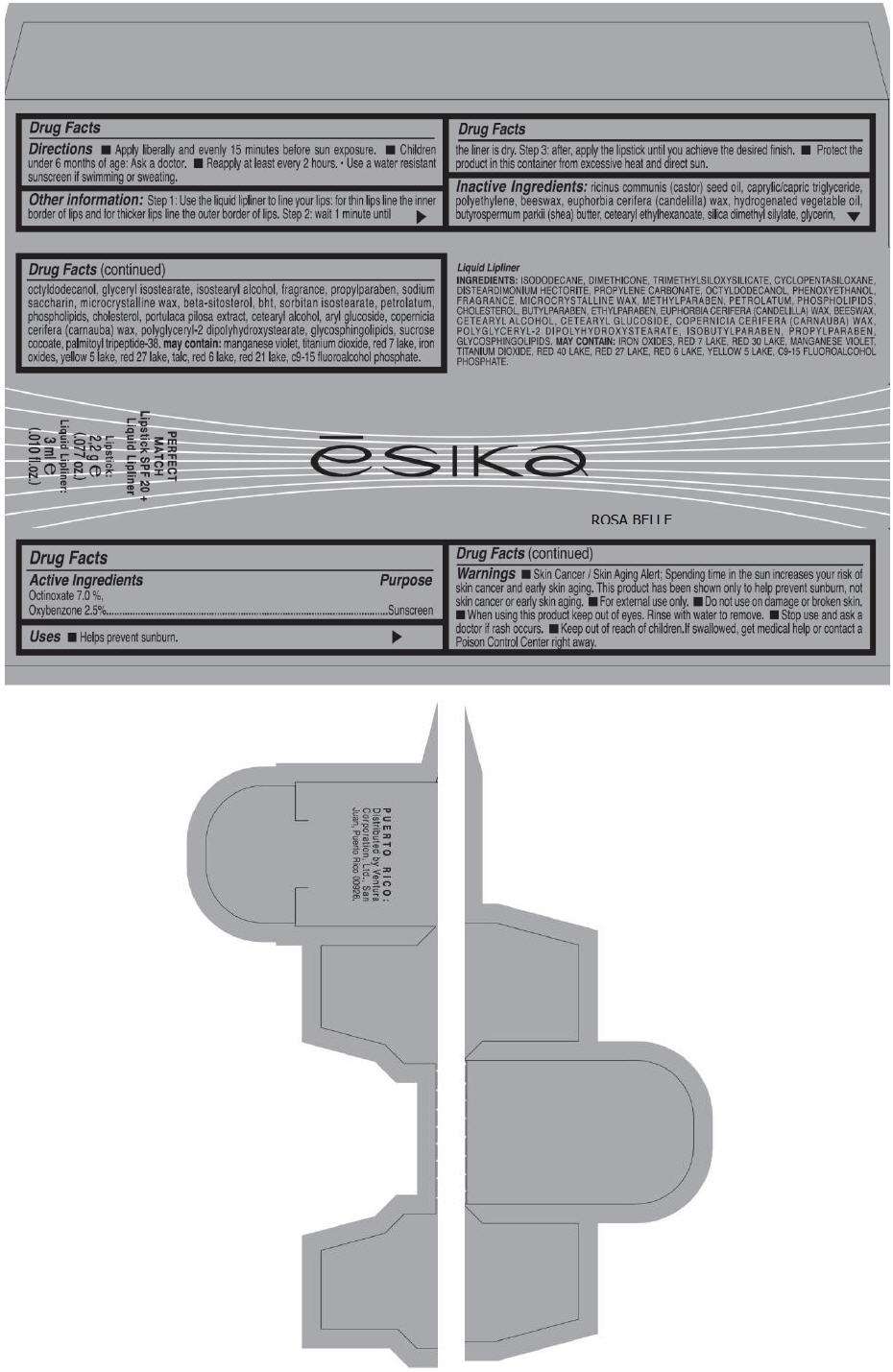
- PRINCIPAL DISPLAY PANEL - 50 g Tube Carton - ROSA BELLE
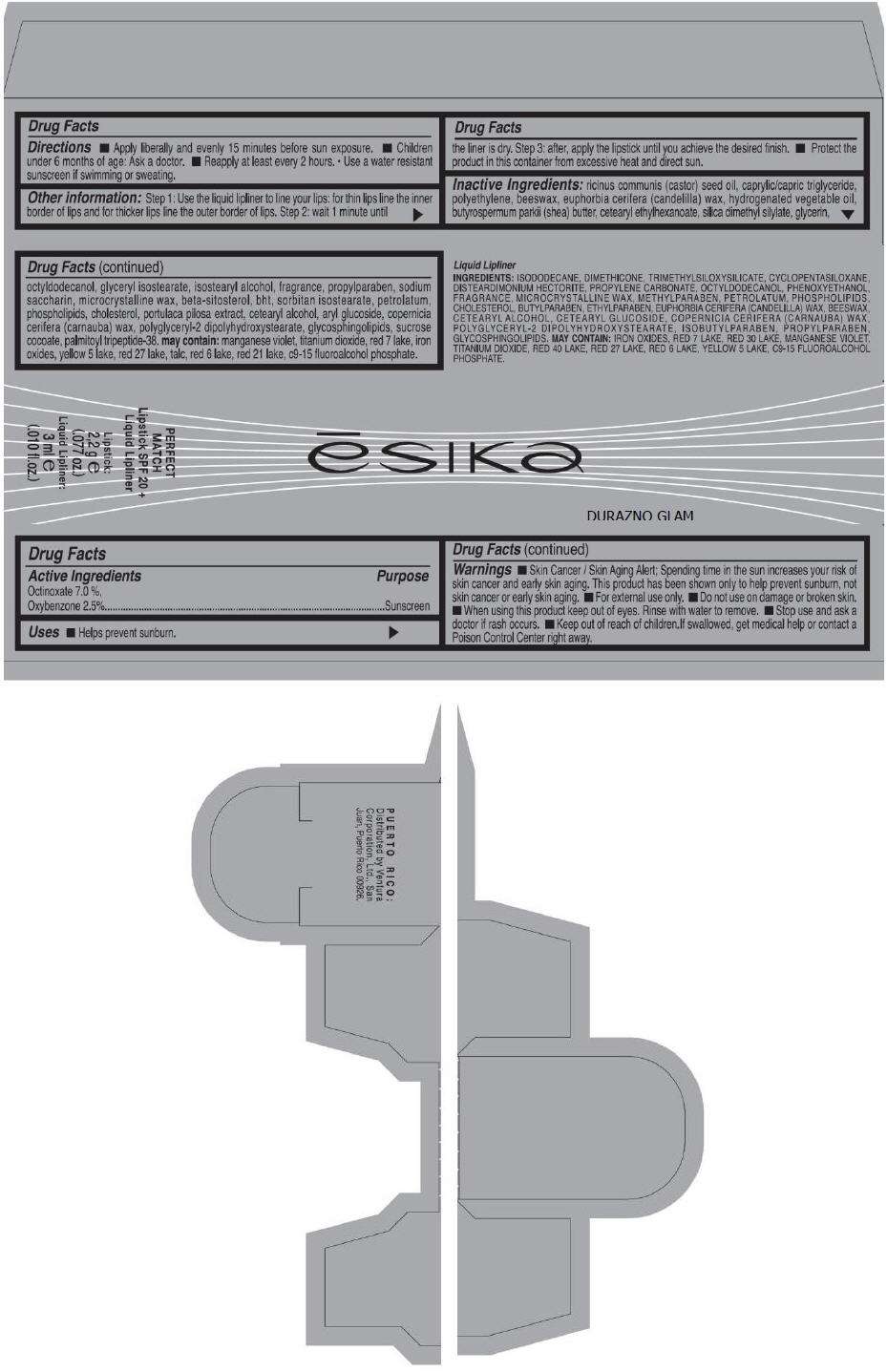
- PRINCIPAL DISPLAY PANEL - 50 g Tube Carton - DURAZNO GLAM
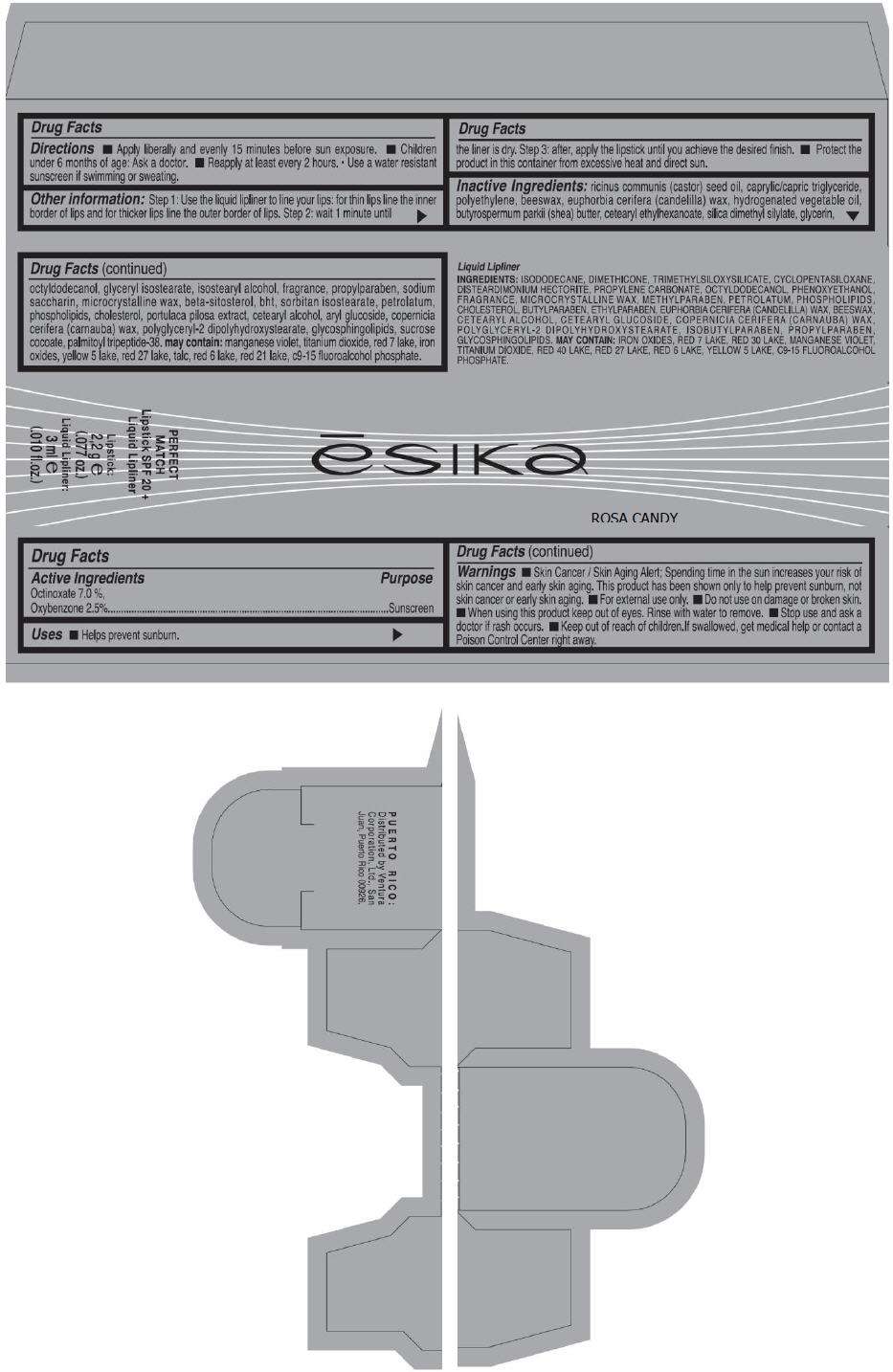
- PRINCIPAL DISPLAY PANEL - 50 g Tube Carton - ROSA CANDY
- PRINCIPAL DISPLAY PANEL - 50 g Tube Carton - CORAL SEDUCTION
- PRINCIPAL DISPLAY PANEL - 50 g Tube Carton - CHERRY CLASS
- PRINCIPAL DISPLAY PANEL - 50 g Tube Carton - RED SUPREME
- PRINCIPAL DISPLAY PANEL - 50 g Tube Carton - VINO MIDNIGHT
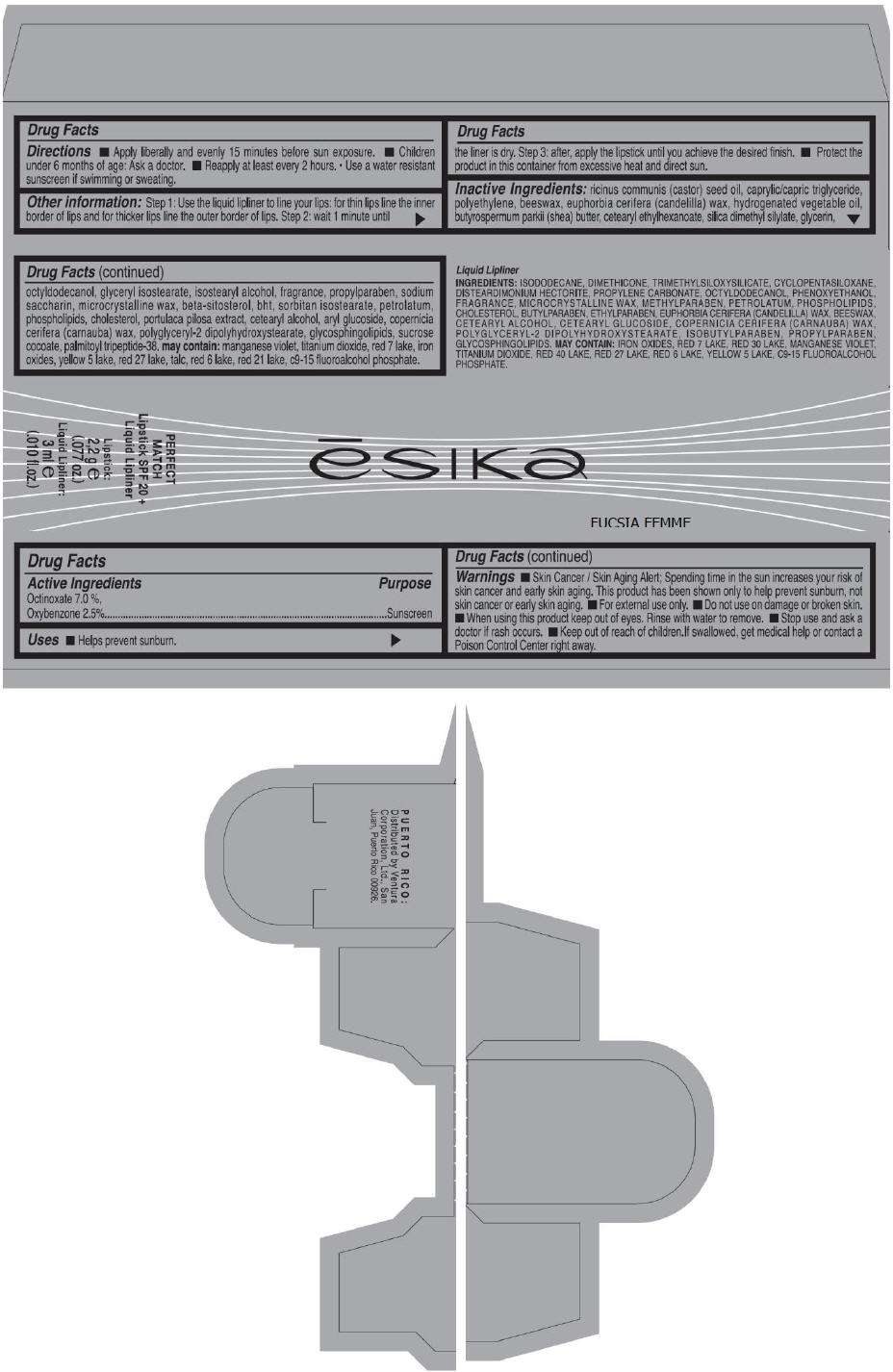
- PRINCIPAL DISPLAY PANEL - 50 g Tube Carton - FUCSIA FEMME
- PRINCIPAL DISPLAY PANEL - 50 g Tube Carton - CEREZA DEEP
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredients
OCTINOXATE 7.0 %,
OXYBENZONE 2.5 %
Purpose
Sunscreen
ESIKA Perfect Match Uses
- Helps prevent sunburn
Warnings
- Skin Cancer / Skin Aging Alert; Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
- For external use only.
- Do not use on damage or broken skin.
- When using this product keep out of eyes. Rinse with water to remove.
- Stop use and ask a doctor if rash occurs.
- Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply liberally and evenly 15 minutes before sun exposure.
- Children under 6 months of age: Ask a doctor.
- Reapply at least every 2 hours.
- Use a water resistant sunscreen if swimming or sweating.
ESIKA Perfect Match Other information
Step 1: Use the liquid lipliner to line your lips: for thin lips line the inner border of lips and for thicker lips line the outer border of lips.
Step 2: wait 1 minute until the liner is dry.
Step 3: after, apply the lipstick until you achieve the desired finish.
- Protect the product in this container from excessive heat and direct sun.
Inactive Ingredients
ricinus communis (castor) seed oil, caprylic/capric triglyceride, polyethylene, beeswax, euphorbia cerifera (candelilla) wax, hydrogenated vegetable oil, butyrospermum parkii (shea) butter, cetearyl ethylhexanoate, silica dimethyl silylate, glycerin,octyldodecanol, glyceryl isostearate, isostearyl alcohol, fragrance, propylparaben, sodium saccharin, microcrystalline wax, beta-sitosterol, bht, sorbitan isostearate, petrolatum, phospholipids, cholesterol, portulaca pilosa extract, cetearyl alcohol, aryl glucoside, copernicia cerifera (carnauba) wax, polyglyceryl-2 dipolyhydroxystearate, glycosphingolipids, sucrose cocoate, palmitoyl tripeptide-38. may contain: manganese violet, titanium dioxide, red 7 lake, iron oxides, yellow 5 lake, red 27 lake, talc, red 6 lake, red 21 lake, c9-15 fluoroalcohol phosphate.
Dist. by Ventura Corporation, Ltd., San Juan, Puerto Rico 00926.
PRINCIPAL DISPLAY PANEL - 50 g Tube Carton - COFFEE SWEET
ēsika
COFFEE SWEET
PERFECT
MATCH
Lipstick SPF 20 +
Liquid Lipliner
Lipstick:
2,2 g e
(.077 oz.)
Liquid Lipliner:
3 ml e
(.010 fl.oz.)
PRINCIPAL DISPLAY PANEL - 50 g Tube Carton - ROSA BELLE
ēsika
ROSA BELLE
PERFECT
MATCH
Lipstick SPF 20 +
Liquid Lipliner
Lipstick:
2,2 g e
(.077 oz.)
Liquid Lipliner:
3 ml e
(.010 fl.oz.)
PRINCIPAL DISPLAY PANEL - 50 g Tube Carton - DURAZNO GLAM
ēsika
DURAZNO GLAM
PERFECT
MATCH
Lipstick SPF 20 +
Liquid Lipliner
Lipstick:
2,2 g e
(.077 oz.)
Liquid Lipliner:
3 ml e
(.010 fl.oz.)
PRINCIPAL DISPLAY PANEL - 50 g Tube Carton - ROSA CANDY
ēsika
ROSA CANDY
PERFECT
MATCH
Lipstick SPF 20 +
Liquid Lipliner
Lipstick:
2,2 g e
(.077 oz.)
Liquid Lipliner:
3 ml e
(.010 fl.oz.)
PRINCIPAL DISPLAY PANEL - 50 g Tube Carton - CORAL SEDUCTION
ēsika
CORAL SEDUCTION
PERFECT
MATCH
Lipstick SPF 20 +
Liquid Lipliner
Lipstick:
2,2 g e
(.077 oz.)
Liquid Lipliner:
3 ml e
(.010 fl.oz.)
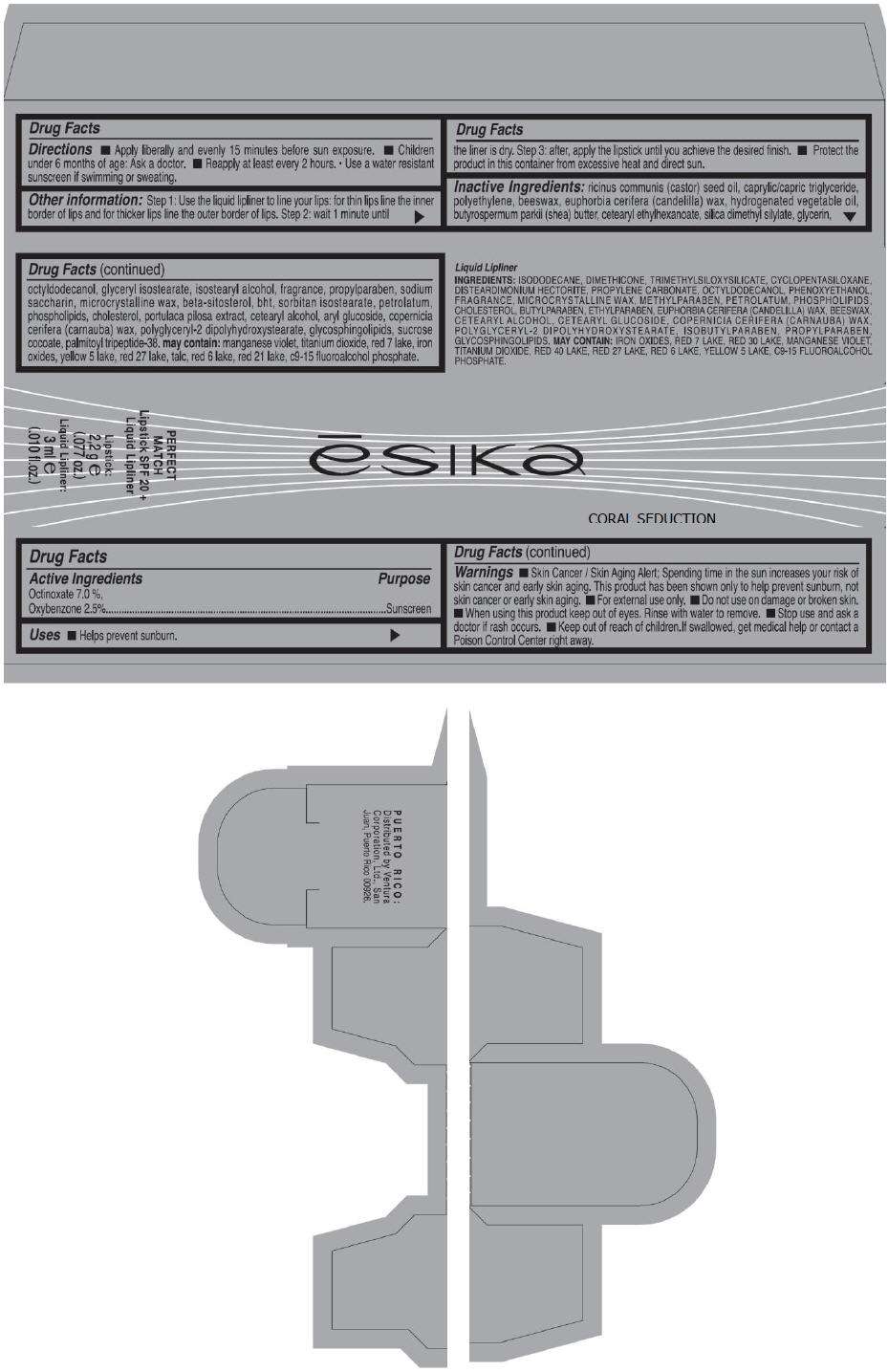
PRINCIPAL DISPLAY PANEL - 50 g Tube Carton - CHERRY CLASS
ēsika
CHERRY CLASS
PERFECT
MATCH
Lipstick SPF 20 +
Liquid Lipliner
Lipstick:
2,2 g e
(.077 oz.)
Liquid Lipliner:
3 ml e
(.010 fl.oz.)
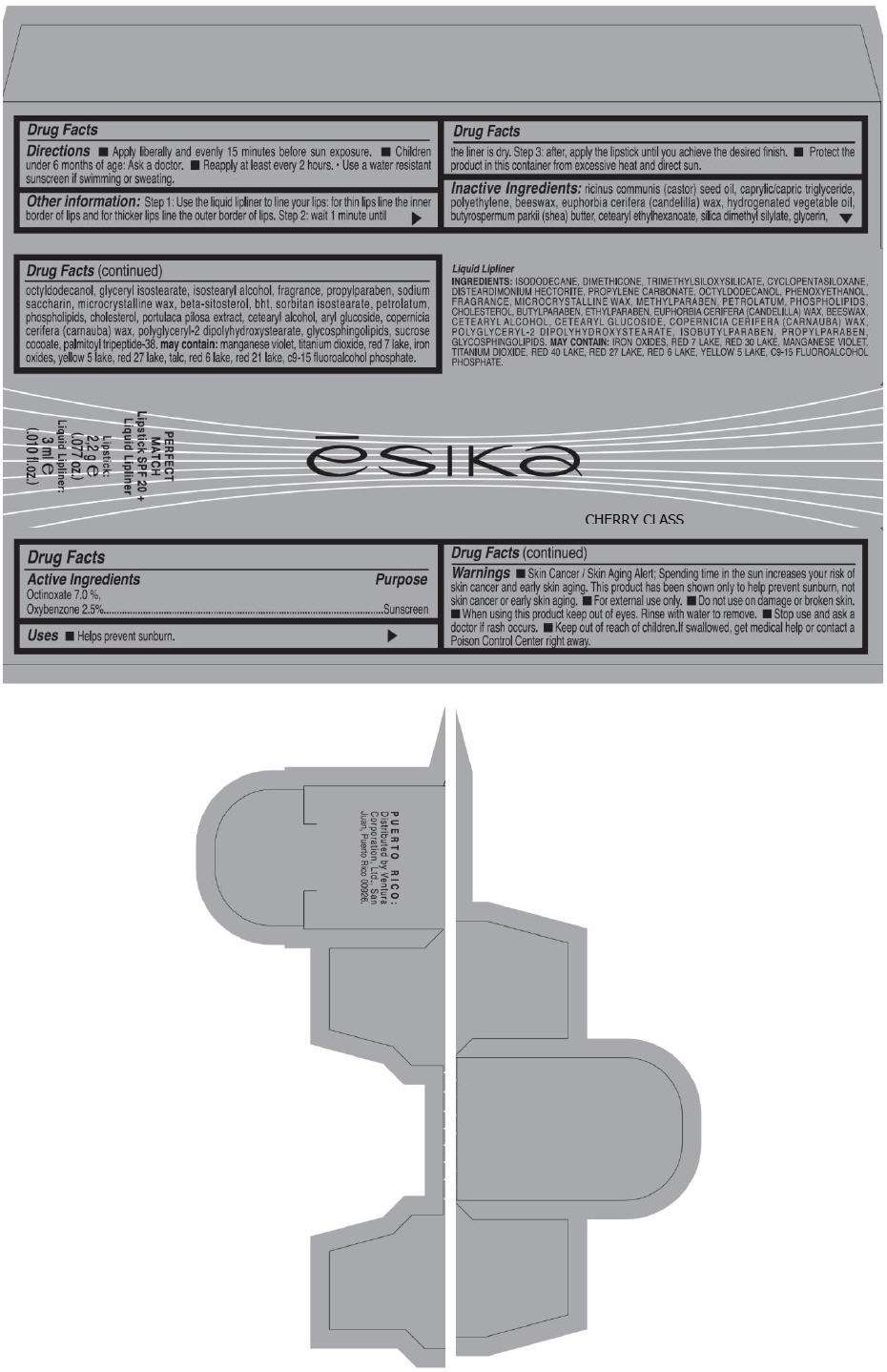
PRINCIPAL DISPLAY PANEL - 50 g Tube Carton - RED SUPREME
ēsika
RED SUPREME
PERFECT
MATCH
Lipstick SPF 20 +
Liquid Lipliner
Lipstick:
2,2 g e
(.077 oz.)
Liquid Lipliner:
3 ml e
(.010 fl.oz.)
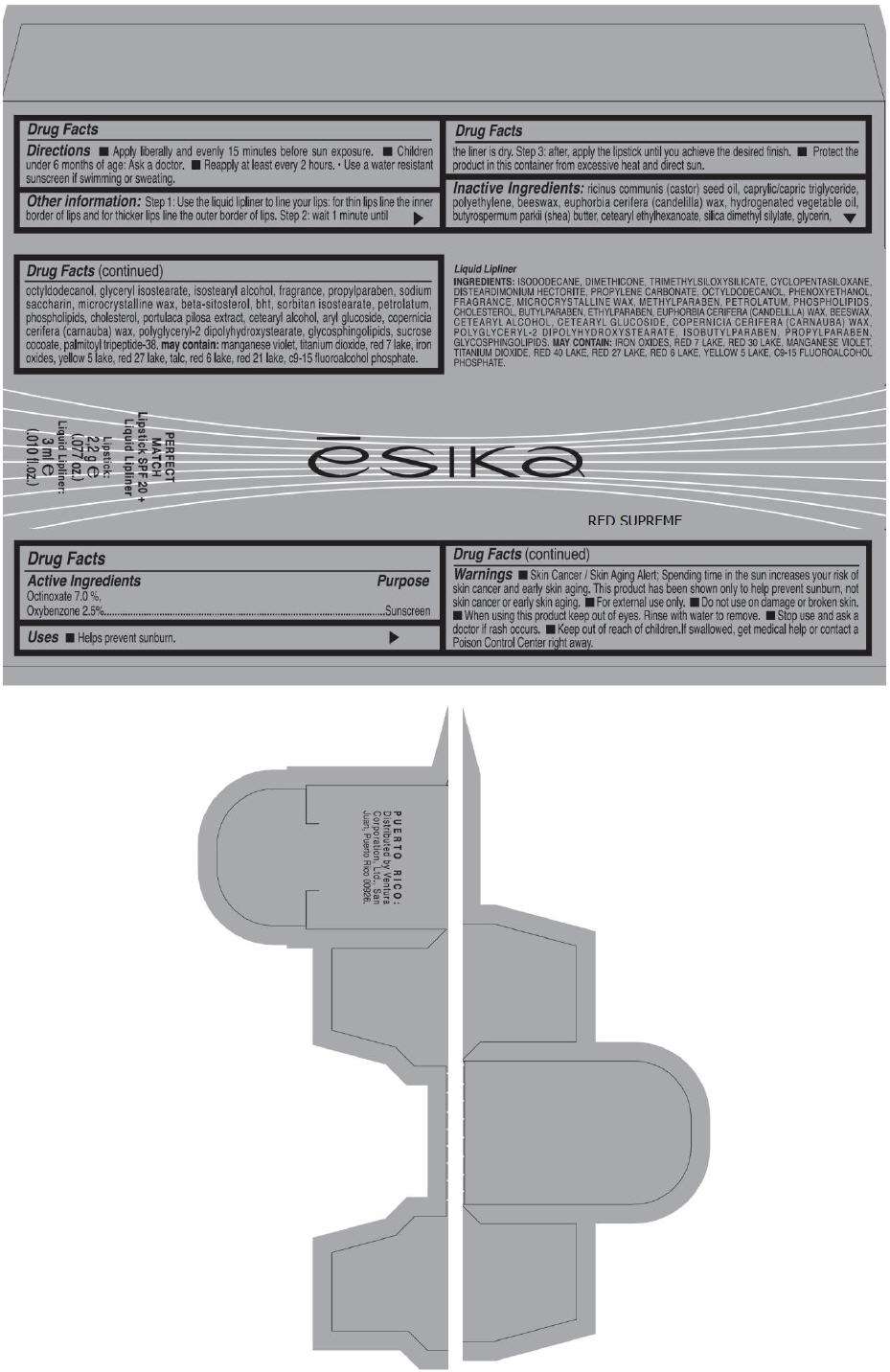
PRINCIPAL DISPLAY PANEL - 50 g Tube Carton - VINO MIDNIGHT
ēsika
VINO MIDNIGHT
PERFECT
MATCH
Lipstick SPF 20 +
Liquid Lipliner
Lipstick:
2,2 g e
(.077 oz.)
Liquid Lipliner:
3 ml e
(.010 fl.oz.)
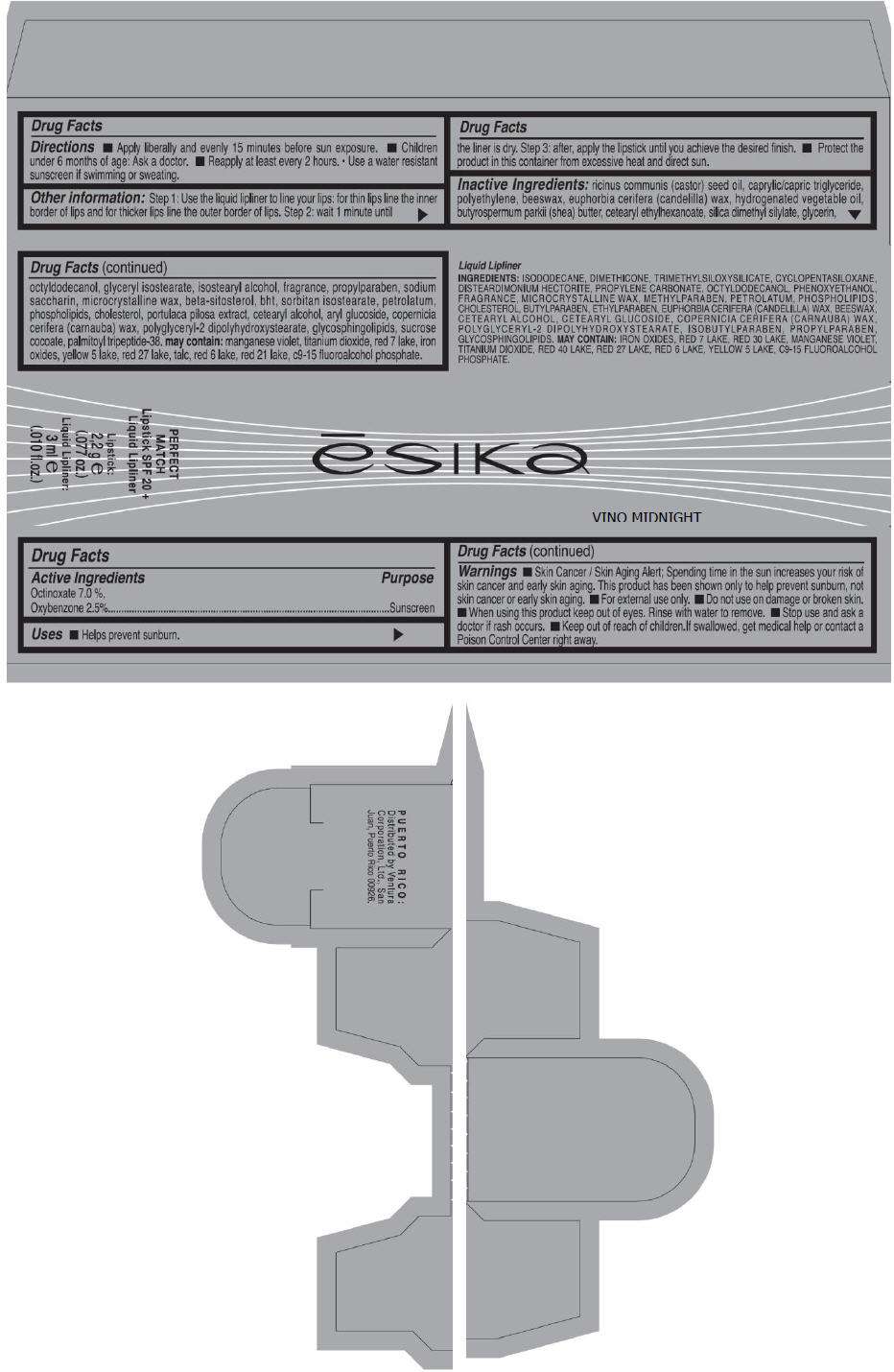
PRINCIPAL DISPLAY PANEL - 50 g Tube Carton - FUCSIA FEMME
ēsika
FUCSIA FEMME
PERFECT
MATCH
Lipstick SPF 20 +
Liquid Lipliner
Lipstick:
2,2 g e
(.077 oz.)
Liquid Lipliner:
3 ml e
(.010 fl.oz.)
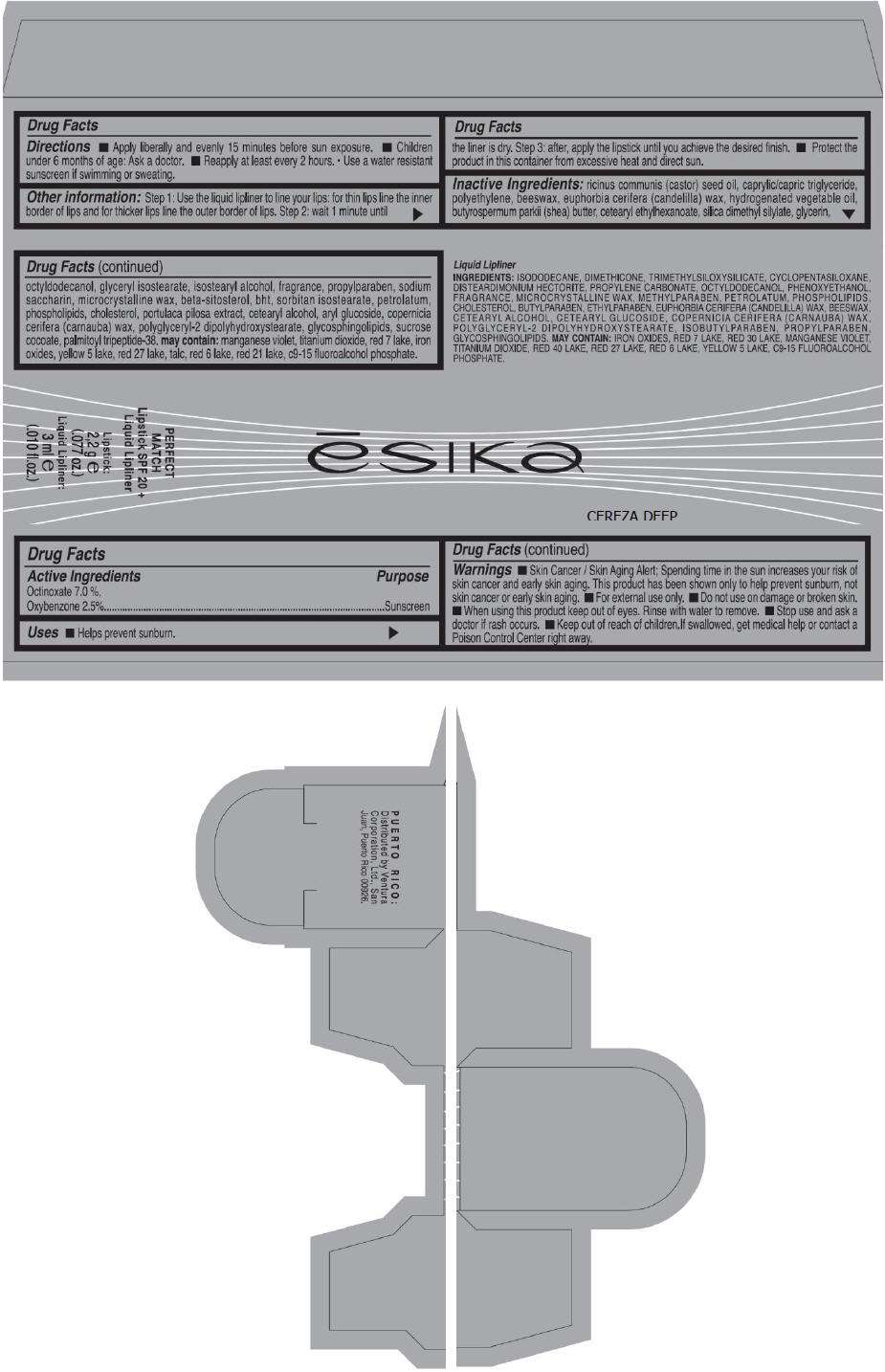
PRINCIPAL DISPLAY PANEL - 50 g Tube Carton - CEREZA DEEP
ēsika
CEREZA DEEP
PERFECT
MATCH
Lipstick SPF 20 +
Liquid Lipliner
Lipstick:
2,2 g e
(.077 oz.)
Liquid Lipliner:
3 ml e
(.010 fl.oz.)
ESIKA Perfect MatchOctinoxate and Oxybenzone LIPSTICK
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
ESIKA Perfect MatchOctinoxate and Oxybenzone LIPSTICK
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
ESIKA Perfect MatchOctinoxate and Oxybenzone LIPSTICK
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
ESIKA Perfect MatchOctinoxate and Oxybenzone LIPSTICK
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
ESIKA Perfect MatchOctinoxate and Oxybenzone LIPSTICK
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
ESIKA Perfect MatchOctinoxate and Oxybenzone LIPSTICK
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
ESIKA Perfect MatchOctinoxate and Oxybenzone LIPSTICK
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
ESIKA Perfect MatchOctinoxate and Oxybenzone LIPSTICK
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
ESIKA Perfect MatchOctinoxate and Oxybenzone LIPSTICK
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
ESIKA Perfect MatchOctinoxate and Oxybenzone LIPSTICK
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||