Ery-Tab
FULL PRESCRIBING INFORMATION: CONTENTS*
- ERY-TAB DESCRIPTION
- INACTIVE INGREDIENT
- CLINICAL PHARMACOLOGY
- MICROBIOLOGY
- INDICATIONS & USAGE
- ERY-TAB CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- ERY-TAB ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
ERY-TAB DESCRIPTION
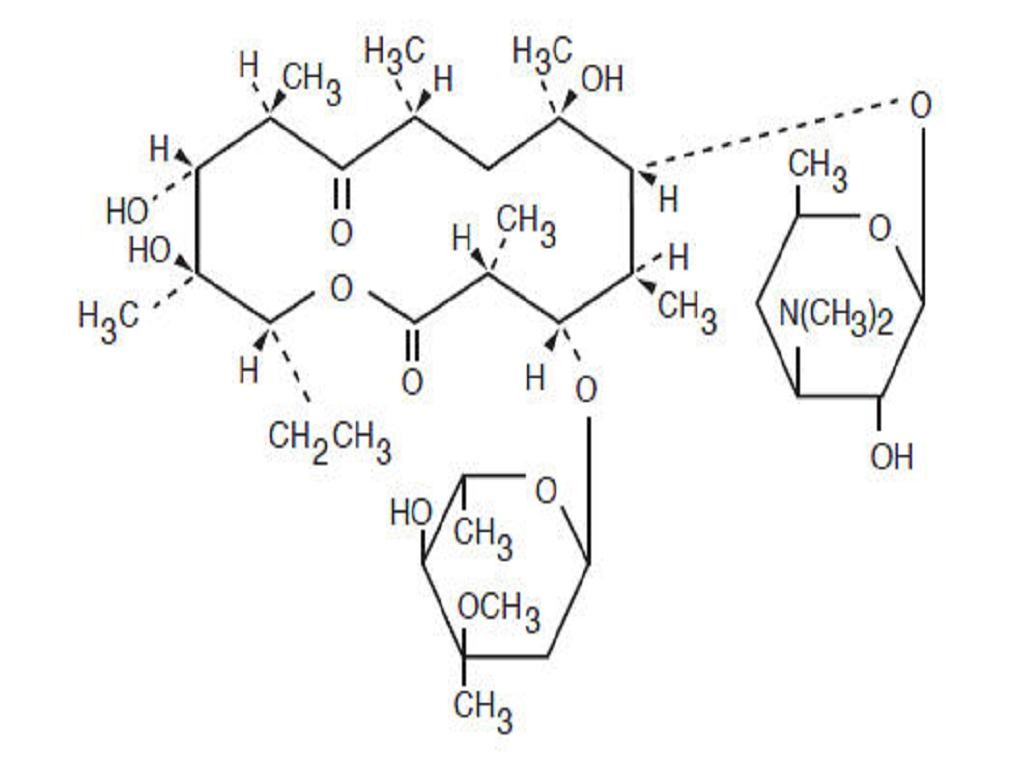
INACTIVE INGREDIENT
CLINICAL PHARMACOLOGY
MICROBIOLOGY
INDICATIONS AND USAGE
Gram-positive Organisms
Gram-negative Organisms
Other Microorganisms
Gram-positive Organisms
Gram-negative Organisms
Susceptibility Tests
Dilution Techniques
MIC (mcg/mL)Interpretation
MicroorganismMIC (mcg/mL)
Diffusion Techniques
Zone Diameter (mm)Interpretation
MicroorganismZone Diameter (mm)
INDICATIONS & USAGE
Prophylaxis
Prevention of Initial Attacks of Rheumatic Fever
Prevention of Recurrent Attacks of Rheumatic Fever
ERY-TAB CONTRAINDICATIONS
PRECAUTIONS - Drug Interactions
WARNINGS
PRECAUTIONS
GeneralCLINICAL PHARMACOLOGYWARNINGS
INFORMATION FOR PATIENTS
DRUG INTERACTIONS
Ergotamine/dihydroergotamine
Triazolobenzodiazepines (such as triazolam and alprazolam) and Related Benzodiazepines
HMG-CoA Reductase Inhibitors
Sildenafil (Viagra)
CONTRAINDICATIONS
CONTRAINDICATIONS
CONTRAINDICATIONS
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic effectsPregnancy Category B
LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
INDICATIONS AND USAGEDOSAGE AND ADMINISTRATIONGERIATRIC USE
ADVERSE REACTIONSDOSAGE AND ADMINISTRATIONADVERSE REACTIONS
PRECAUTIONS - Drug Interactions
ERY-TAB ADVERSE REACTIONS
WARNINGSWARNINGS
OVERDOSAGE
DOSAGE & ADMINISTRATION
Adults
Children
Conjunctivitis of the Newborn Caused by Chlamydia trachomatis
Pneumonia of Infancy Caused by Chlamydia trachomatis
Urogenital Infections During Pregnancy Due to Chlamydia trachomatis
For Adults With Uncomplicated Urethral, Endocervical, or Rectal Infections Caused by Chlamydia trachomatis, When Tetracycline is Contraindicated or Not Tolerated
For Patients With Nongonococcal Urethritis Caused by Ureaplasma Urealyticum When Tetracycline is Contraindicated or Not Tolerated
Primary Syphilis
Acute Pelvic Inflammatory Disease Caused by N. Gonorrhoeae
Intestinal Amebiasis
Pertussis
Legionnaires' Disease
Preoperative Prophylaxis for Elective Colorectal Surgery
Pre-op Day 3
Pre-op Day 2
Pre-op Day 1
Day of Operation
HOW SUPPLIED
STORAGE AND HANDLING
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Ery-TabErythromycin TABLET, DELAYED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!