ERIVEDGE
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ERIVEDGE safely and effectively. See full prescribing information for ERIVEDGE. ERIVEDGE (vismodegib) capsule for oral useInitial U.S. Approval: 2012BOXED WARNINGWARNING: EMBRYO-FETAL DEATH AND SEVERE BIRTH DEFECTS See full prescribing information for complete boxed warning. ERIVEDGE can result in embryo-fetal death or severe birth defects. Verify pregnancy status prior to initiation of ERIVEDGE. Advise male and female patients of these risks. Advise females of the need for contraception and advise males of the potential risk of ERIVEDGE exposure through semen. (Boxed Warning, 5.1, 8.1, 8.6)INDICATIONS AND USAGEERIVEDGE® (vismodegib) capsule is a hedgehog pathway inhibitor indicated for the treatment of adults with metastatic basal cell carcinoma, or with locally advanced basal cell carcinoma that has recurred following surgery or who are not candidates for surgery, and who are not candidates for radiation. (1)DOSAGE AND ADMINISTRATIONThe recommended dose is 150 mg orally once daily. (2)DOSAGE FORMS AND STRENGTHS150 mg capsules. (3)CONTRAINDICATIONSNone.WARNINGS AND PRECAUTIONS Embryo-fetal death and severe birth defects: ERIVEDGE can cause embryo-fetal death or severe birth defects. (5.1) Blood donation: Advise patients not to donate blood or blood products while receiving ERIVEDGE and for at least 7 months after the last dose of ERIVEDGE. (5.2) Side Effects The most common adverse reactions (incidence of ≥ 10%) are muscle spasms, alopecia, dysgeusia, weight loss, fatigue, nausea, diarrhea, decreased appetite, constipation, arthralgias, vomiting, and ageusia. To report SUSPECTED ADVERSE REACTIONS, contact Genentech, Inc. at 1-888-835-2555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. USE IN SPECIFIC POPULATIONS Pregnancy: Can cause fetal harm. Advise females of reproductive potential of potential risk to the fetus. (5.1, 8.1) Nursing Mothers: Discontinue drug or nursing taking into consideration importance of drug to mother. (8.3) Females of Reproductive Potential and Males: Counsel males and females on pregnancy prevention and planning. Report immediately exposure to ERIVEDGE during pregnancy to the Genentech Adverse Event Line at 1-888-835-2555. Encourage patient participation in the ERIVEDGE pregnancy pharmacovigilance program by contacting the Genentech Adverse Event Line at 1-888-835-2555. (8.6)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 ERIVEDGE INDICATIONS AND USAGE
- 2 ERIVEDGE DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 ERIVEDGE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 ERIVEDGE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 ERIVEDGE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
FULL PRESCRIBING INFORMATION
ERIVEDGE (vismodegib) capsule can result in embryo-fetal death or severe birth defects. ERIVEDGE is embryotoxic and teratogenic in animals. Teratogenic effects included severe midline defects, missing digits, and other irreversible malformations.
Verify pregnancy status prior to the initiation of ERIVEDGE. Advise male and female patients of these risks. Advise female patients of the need for contraception and advise male patients of the potential risk of ERIVEDGE exposure through semen [see Warnings and Precautions (5.1), Use in Specific Populations (8.1, 8.6) ].
1 INDICATIONS AND USAGE
ERIVEDGE capsule is indicated for the treatment of adults with metastatic basal cell carcinoma, or with locally advanced basal cell carcinoma that has recurred following surgery or who are not candidates for surgery, and who are not candidates for radiation.
2 DOSAGE AND ADMINISTRATION
The recommended dose of ERIVEDGE is 150 mg taken orally once daily until disease progression or until unacceptable toxicity [see Clinical Studies (14) ].
ERIVEDGE may be taken with or without food. Swallow capsules whole. Do not open or crush capsules.
If a dose of ERIVEDGE is missed, do not make up that dose; resume dosing with the next scheduled dose.
3 DOSAGE FORMS AND STRENGTHS
ERIVEDGE (vismodegib) capsules, 150 mg. The capsule has a pink opaque body and a grey opaque cap, with "150 mg" printed on the capsule body and "VISMO" printed on the capsule cap in black ink.
4 CONTRAINDICATIONS
None.
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Death and Severe Birth Defects
ERIVEDGE capsules can cause fetal harm when administered to a pregnant woman based on its mechanism of action. Vismodegib is teratogenic, embryotoxic, and fetotoxic in rats at maternal exposures lower than the human exposures at the recommended dose of 150 mg/day. In rats, malformations included craniofacial anomalies, open perineum, and absent or fused digits. Fetal retardations and variations were also observed.
Verify pregnancy status prior to the initiation of ERIVEDGE. Advise male and female patients of the risks of embryo-fetal death and severe birth defects and the need for contraception during and after treatment. Advise patients to contact their healthcare provider immediately if they suspect they (or, for males, their female partner) may be pregnant. Female and male patients of reproductive potential should be counseled regarding pregnancy prevention and planning. If ERIVEDGE is used during pregnancy or if a patient becomes pregnant while taking (or for a male patient, if his female partner is exposed to) ERIVEDGE, the patient should be apprised of the potential hazard to the fetus. Report immediately exposure to ERIVEDGE during pregnancy to the Genentech Adverse Event Line at 1-888-835-2555. Encourage women who may have been exposed to ERIVEDGE during pregnancy, either directly or through seminal fluid, to participate in the ERIVEDGE pregnancy pharmacovigilance program by contacting the Genentech Adverse Event Line at 1-888-835-2555 [see Boxed Warning, Use in Specific Populations (8.1, 8.6) ].
5.2 Blood Donation
Advise patients not to donate blood or blood products while receiving ERIVEDGE and for at least 7 months after the last dose of ERIVEDGE.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
ERIVEDGE capsule was administered as monotherapy at doses ≥ 150 mg orally daily in four open-label, uncontrolled, dose-ranging or fixed single dose clinical trials enrolling a total of 138 patients with advanced basal cell carcinoma (BCC). The median age of these patients was 61 years (range 21 to 101), 100% were White (including Hispanics), and 64% were male. The median duration of treatment was approximately 10 months (305 days; range 0.7 to 36 months); 111 patients received ERIVEDGE for 6 months or longer.
The most common adverse reactions (≥ 10%) were muscle spasms, alopecia, dysgeusia, weight loss, fatigue, nausea, diarrhea, decreased appetite, constipation, arthralgias, vomiting, and ageusia (Table 1).
| All aBCC |
|||
|---|---|---|---|
| MedDRA Preferred Term |
All Grades |
Grade 3 (%) | Grade 4 (%) |
| Gastrointestinal disorders | |||
| Nausea | 42 (30.4%) | 1 (0.7%) | - |
| Diarrhea | 40 (29.0%) | 1 (0.7%) | - |
| Constipation | 29 (21.0%) | - | - |
| Vomiting | 19 (13.8%) | - | - |
| General disorders and administration site conditions | |||
| Fatigue | 55 (39.9%) | 7 (5.1%) | 1 (0.7%) |
| Investigations | |||
| Weight loss | 62 (44.9%) | 10 (7.2%) | - |
| Metabolism and nutrition disorders | |||
| Decreased appetite | 35 (25.4%) | 3 (2.2%) | - |
| Musculoskeletal and connective tissue disorders | |||
| Muscle spasms | 99 (71.7%) | 5 (3.6%) | - |
| Arthralgias | 22 (15.9%) | 1 (0.7%) | |
| Nervous system disorders | |||
| Dysgeusia | 76 (55.1%) | - | - |
| Ageusia | 15 (10.9%) | - | - |
| Skin and subcutaneous tissue disorders | |||
| Alopecia | 88 (63.8%) | - | - |
Amenorrhea:
In clinical trials, a total of 3 of 10 pre-menopausal women developed amenorrhea while receiving ERIVEDGE [see Non-Clinical Toxicology (13.1) ].
Laboratory Abnormalities:
Treatment-emergent Grade 3 laboratory abnormalities observed in clinical trials were hyponatremia in 6 patients (4%), hypokalemia in 2 patients (1%), and azotemia in 3 patients (2%).
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on Vismodegib
Drugs that Inhibit or Induce Drug Metabolizing Enzymes
Vismodegib elimination involves multiple pathways. Vismodegib is predominantly excreted as an unchanged drug. Several minor metabolites are produced by multiple CYP enzymes. Although vismodegib is a substrate of CYP2C9 and CYP3A4 in vitro, CYP inhibition is not predicted to alter vismodegib systemic exposure since similar steady-state plasma vismodegib concentrations were observed in patients in clinical trials concomitantly treated with CYP3A4 inducers (i.e., carbamazepine, modafinil, phenobarbital) and those concomitantly treated with CYP3A4 inhibitors (i.e., erythromycin, fluconazole).
Drugs that Inhibit Drug Transport Systems
In vitro studies indicate that vismodegib is a substrate of the efflux transporter P-glycoprotein (P-gp). When ERIVEDGE is coadministered with drugs that inhibit P-gp (e.g. clarithromycin, erythromycin, azithromycin), systemic exposure of vismodegib and incidence of adverse events of ERIVEDGE may be increased.
Drugs that Affect Gastric pH
Drugs that alter the pH of the upper GI tract (e.g. proton pump inhibitors, H2-receptor antagonists, and antacids) may alter the solubility of vismodegib and reduce its bioavailability. However, no formal clinical study has been conducted to evaluate the effect of gastric pH altering agents on the systemic exposure of vismodegib. Increasing the dose of ERIVEDGE when coadministered with such agents is not likely to compensate for the loss of exposure. When ERIVEDGE is coadministered with a proton pump inhibitor, H2-receptor antagonist or antacid, systemic exposure of vismodegib may be decreased and the effect on efficacy of ERIVEDGE is unknown.
7.2 Effects of Vismodegib on Other Drugs
Results of a drug-drug interaction study conducted in cancer patients demonstrated that the systemic exposure of rosiglitazone (a CYP2C8 substrate) or oral contraceptives (ethinyl estradiol and norethindrone) is not altered when either drug is co-administered with vismodegib.
In vitro studies indicate that vismodegib is an inhibitor of CYP2C8, CYP2C9, CYP2C19 and the transporter BCRP. Vismodegib does not induce CYP1A2, CYP2B6, or CYP3A4/5 in human hepatocytes.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category D
ERIVEDGE capsule can cause fetal harm when administered to a pregnant female based on its mechanism of action. Vismodegib is teratogenic in rats at doses corresponding to an exposure of 20% of the exposure at the recommended human dose (estimated AUC0-24hr steady-state exposure). In rats, malformations included craniofacial anomalies, open perineum, and absent or fused digits. Fetal retardations and variations were also observed. Vismodegib is embryolethal in rats at exposures within the range achieved at the recommended human dose. If ERIVEDGE is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the embryo or fetus. Report immediately exposure to ERIVEDGE during pregnancy to the Genentech Adverse Event Line at 1-888-835-2555. Encourage women who may have been exposed to ERIVEDGE during pregnancy, either directly or through seminal fluid, to participate in the ERIVEDGE pregnancy pharmacovigilance program by contacting the Genentech Adverse Event Line at 1-888-835-2555 [see Boxed Warning, Warnings and Precautions (5.1) ].
In an embryo-fetal developmental toxicity study, pregnant rats were administered oral vismodegib at doses of 10, 60, or 300 mg/kg/day during the period of organogenesis. Pre- and post-implantation loss were increased at doses of ≥ 60 mg/kg/day (approximately ≥ 2 times the systemic exposure (AUC) in patients at the recommended human dose), which included early resorption of 100% of the fetuses. A dose of 10 mg/kg/day (approximately 0.2 times the AUC in patients at the recommended dose) resulted in malformations (including missing and/or fused digits, open perineum and craniofacial anomalies) and retardations or variations (including dilated renal pelvis, dilated ureter, and incompletely or unossified sternal elements, centra of vertebrae, or proximal phalanges and claws).
8.3 Nursing Mothers
It is not known whether vismodegib is excreted in human breast milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from ERIVEDGE, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
The safety and effectiveness of ERIVEDGE capsule have not been established in pediatric patients.
In repeat-dose toxicology studies in rats, administration of oral vismodegib resulted in toxicities in bone and teeth. Effects on bone consisted of closure of the epiphyseal growth plate when oral vismodegib was administered for 26 weeks at ≥ 50 mg/kg/day (approximately ≥ 0.4 times the systemic exposure (AUC) in patients at the recommended human dose). Abnormalities in growing incisor teeth (including degeneration/necrosis of odontoblasts, formation of fluid-filled cysts in the dental pulp, ossification of the root canal, and hemorrhage resulting in breakage or loss of teeth) were observed after administration of oral vismodegib at ≥ 15 mg/kg/day (approximately ≥ 0.2 times the AUC in patients at the recommended human dose).
8.5 Geriatric Use
Clinical studies of ERIVEDGE capsule did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients.
8.6 Females of Reproductive Potential and Males
ERIVEDGE capsule can cause harm to the embryo or fetus when administered during pregnancy. Counsel female and male patients regarding pregnancy prevention and planning. Advise patients to contact their healthcare provider immediately if they suspect they (or, for males, their female partner) may be pregnant [see Boxed Warning, Warnings and Precautions (5.1), Use in Specific Populations (8.1) ].
Female patients
Determine pregnancy status within 7 days prior to initiation of treatment in females of reproductive potential. For females with a negative pregnancy test, initiate a highly effective form of contraception (failure rate of less than 1%) prior to the first dose. Continue highly effective contraception during therapy and for 7 months after the last dose of ERIVEDGE. If a patient becomes pregnant while taking ERIVEDGE, or during the 7 months after the last dose of treatment, report the pregnancy to the Genentech Adverse Event Line at 1-888-835-2555. Encourage pregnant females to participate in the ERIVEDGE pregnancy pharmacovigilance program by calling the Genentech Adverse Event Line at 1-888-835-2555. Counsel pregnant females about the teratogenic risk to the fetus.
Amenorrhea has been observed in clinical trials in females of reproductive potential. Reversibility of amenorrhea is unknown [see Adverse Reactions (6), Nonclinical Toxicology (13.1) ].
Male patients
Male patients should use condoms with spermicide, even after a vasectomy, during sexual intercourse with female partners while being treated with ERIVEDGE capsule and for 2 months after the last dose to avoid exposing an embryo or fetus to vismodegib.
8.7 Hepatic Impairment
The safety and effectiveness of ERIVEDGE capsule have not been established in patients with hepatic impairment [see Clinical Pharmacology (12.3) ].
8.8 Renal Impairment
The safety and effectiveness of ERIVEDGE capsule have not been established in patients with renal impairment [see Clinical Pharmacology (12.3) ].
10 OVERDOSAGE
There is no information on overdosage in humans. In clinical trials, ERIVEDGE capsule was administered at 540 mg orally once daily; exposure did not increase between 150 mg and 540 mg daily.
11 DESCRIPTION
Vismodegib is an inhibitor of the hedgehog (Hh) signaling pathway, which is described chemically as 2-Chloro-N-(4-chloro-3-(pyridin-2-yl)phenyl)-4-(methylsulfonyl)benzamide. The molecular formula is C19H14Cl2N2O3S. The molecular weight is 421.30 g/mol and the structural formula is:
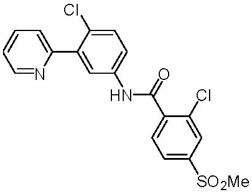
Vismodegib is a crystalline free base with a pKa (pyridinium cation) of 3.8, appearing as a white to tan powder. The solubility of vismodegib is pH dependent with 0.1 μg/mL at pH 7 and 0.99 mg/mL at pH 1. The partition coefficient (log P) is 2.7.
Each ERIVEDGE (vismodegib) capsule for oral administration contains 150 mg vismodegib and the following inactive ingredients: microcrystalline cellulose, lactose monohydrate, sodium lauryl sulfate, povidone, sodium starch glycolate, talc, and magnesium stearate (non-bovine). The capsule shell contains gelatin, titanium dioxide, red iron oxide, and black iron oxide. The black printing ink contains shellac and black iron oxide.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Vismodegib is an inhibitor of the Hedgehog pathway. Vismodegib binds to and inhibits Smoothened, a transmembrane protein involved in Hedgehog signal transduction.
12.3 Pharmacokinetics
Absorption
Vismodegib is a highly permeable compound with low aqueous solubility (BCS Class 2). The single dose absolute bioavailability of vismodegib is 31.8%. Absorption is saturable as evidenced by the lack of dose proportional increase in exposure after a single dose of 270 mg or 540 mg vismodegib. ERIVEDGE capsule may be taken without regard to meals because the systemic exposure of vismodegib at steady state is not affected by food.
Distribution
The volume of distribution of vismodegib ranges from 16.4 to 26.6 L. Vismodegib plasma protein binding in patients is greater than 99%. Vismodegib binds to both human serum albumin and alpha-1-acid glycoprotein (AAG) and binding to AAG is saturable.
Metabolism
Greater than 98% of the total circulating drug-related components are the parent drug. Metabolic pathways of vismodegib in humans include oxidation, glucuronidation, and pyridine ring cleavage. The two most abundant oxidative metabolites recovered in feces are produced in vitro by recombinant CYP2C9 and CYP3A4/5.
Elimination
Vismodegib and its metabolites are eliminated primarily by the hepatic route with 82% of the administered dose recovered in the feces and 4.4% recovered in urine. The estimated elimination half-life (t1/2) of vismodegib is 4 days after continuous once-daily dosing and 12 days after a single dose.
Pharmacokinetics in Specific Populations
Hepatic Impairment: The effect of hepatic impairment on the systemic exposure of vismodegib has not been studied.
Renal Impairment: The effect of renal impairment on the systemic exposure of vismodegib has not been studied.
Population pharmacokinetic analyses showed that weight (range: 41-140 kg), age (range: 26-89 years), creatinine clearance (range: 30 to 80 mL/min), and sex do not have a clinically meaningful influence on the systemic exposure of vismodegib.
12.6 Cardiac Electrophysiology
In a thorough QTc study in 60 healthy subjects, there was no effect of therapeutic doses of ERIVEDGE on the QTc interval.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies with vismodegib have not been conducted. Pilomatricoma (a benign cutaneous neoplasm) was observed in rats administered oral vismodegib for 26 weeks at 100 mg/kg/day (approximately 0.8 times the systemic exposure (AUC) in patients at the recommended human dose).
Vismodegib was not mutagenic in the in vitro bacterial reverse mutation (Ames) assay and was not clastogenic in the in vitro human chromosomal aberration assay in human peripheral blood lymphocytes or in the in vivo rat bone marrow micronucleus assay.
Studies to assess the potential of vismodegib to affect fertility have not been conducted; however, data from repeat-dose toxicology studies in rats and dogs indicate that male and female reproductive function and fertility may be impaired in patients receiving ERIVEDGE capsule. In a 26-week toxicology study in rats, a relative decrease in percent motile sperm was observed at ≥ 15 mg/kg/day (approximately ≥ 0.3 times the AUC in patients at the recommended human dose). In dogs, increased numbers of degenerating germ cells and hypospermia were observed in young animals administered oral vismodegib for 4 weeks at ≥ 50 mg/kg/day (approximately ≥ 2 times the AUC in patients at the recommended human dose). No corresponding findings were observed in sexually mature dogs at similar doses in 13-week and 26-week toxicology studies. A decrease in the number of corpora lutea was observed in female rats administered oral vismodegib for 26 weeks at 100 mg/kg/day (approximately 0.8 times the AUC in patients at the recommended human dose).
13.2 Animal Toxicology
Neurologic effects characterized as limb or body tremors or twitching were observed in rats administered oral vismodegib for 4 weeks or longer at ≥ 50 mg/kg/day (approximately ≥ 0.4 times the AUC in patients at the recommended human dose). These observations resolved upon discontinuation of dosing and were not associated with microscopic findings.
14 CLINICAL STUDIES
A single, international, single-arm, multi-center, open-label, 2-cohort trial was conducted in 104 patients with either metastatic basal cell carcinoma (mBCC) (n = 33) or locally advanced BCC (laBCC) (n = 71). Patients with laBCC were required to have lesions that had recurred after radiotherapy, unless radiotherapy was contraindicated or inappropriate (e.g. Gorlin syndrome; limitations because of location of tumor or cumulative prior radiotherapy dose), and where the lesions were either unresectable or surgical resection would result in substantial deformity. Patients were to receive 150 mg vismodegib per day orally until disease progression or unacceptable toxicity.
The major efficacy outcome measure of the trial was objective response rate (ORR) as assessed by an independent review facility (IRF). In the mBCC cohort, tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0. In the laBCC cohort, tumor response evaluation included measurement of externally assessable tumor (including scar) and assessment for ulceration in photographs, radiographic assessment of target lesions (if appropriate), and tumor biopsy. An objective response in laBCC required at least one of the following criteria and absence of any criterion for disease progression: (1) ≥ 30% reduction in lesion size [sum of the longest diameter (SLD)] from baseline in target lesions by radiographic assessment; (2) ≥ 30% reduction in SLD from baseline in externally visible dimension of target lesions; (3) complete resolution of ulceration in all target lesions. Complete response was defined as objective response (as defined above) with no residual BCC on sampling tumor biopsy. Disease progression was defined as any of the following: (1) ≥ 20% increase in the SLD from nadir in target lesions (either by radiography or by externally visible dimension); (2) new ulceration of target lesions persisting without evidence of healing for at least 2 weeks; (3) new lesions by radiographic assessment or physical examination; (4) progression of non-target lesions by RECIST.
Of the 104 patients enrolled, 96 patients were evaluable for ORR. Twenty-one percent of patients carried a diagnosis of Gorlin syndrome. The median age of the efficacy evaluable population was 62 years (46% were at least 65 years old), 61% male and 100% White. For the mBCC cohort (n = 33), 97% of patients had prior therapy including surgery (97%), radiotherapy (58%), and systemic therapies (30%). For the laBCC cohort (n = 63), 94% of patients had prior therapies including surgery (89%), radiotherapy (27%), and systemic/topical therapies (11%). The median duration of treatment was 10.2 months (range 0.7 to 18.7 months).
The key outcome measures are presented in Table 2, below.
| mBCC (n = 33) |
laBCC (n = 63) |
|
|---|---|---|
|
IRF |
10 (30.3) | 27 (42.9) |
| (95%CI) | (15.6, 48.2) | (30.5, 56.0) |
|
Complete response |
0 (0.0) |
13 (20.6) |
|
Partial response |
10 (30.3) |
14 (22.2) |
|
Median Response Duration (months) |
7.6 |
7.6 |
|
(95% CI |
(5.6, NE |
(5.7, 9.7) |
16 HOW SUPPLIED/STORAGE AND HANDLING
Each ERIVEDGE (vismodegib) capsule has a pink opaque body and a grey opaque cap with "150 mg" printed on the capsule body and "VISMO" printed on the capsule cap in black ink. ERIVEDGE capsules are available in bottles of 28 capsules (NDC 50242-140-01).
Store at room temperature 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Medication Guide).
- Advise patients that ERIVEDGE exposure during pregnancy can cause embryo-fetal death or severe birth defects.
- Instruct female patients of reproductive potential to use a highly effective form of contraception (failure rate of less than 1%) while taking ERIVEDGE and for at least 7 months after the last dose of ERIVEDGE.
- Instruct all male patients, even those with prior vasectomy, to use condoms with spermicide, during sexual intercourse with female partners while taking ERIVEDGE and for at least 2 months after the last dose of ERIVEDGE.
- Instruct patients to immediately contact their healthcare provider if they (or, for males, their female partner) become pregnant or if pregnancy is suspected following exposure to ERIVEDGE.
- Instruct patients to immediately report any pregnancy exposure to ERIVEDGE and encourage participation in the ERIVEDGE pregnancy pharmacovigilance program by calling the Genentech Adverse Event Line at 1-888-835-2555.
- Inform female patients of the potential for serious adverse reactions in nursing infants from ERIVEDGE, taking into account the importance of the drug to the mother.
- Advise patients not to donate blood or blood products while taking ERIVEDGE and for at least 7 months after the last dose of ERIVEDGE.
- Advise patients to swallow ERIVEDGE capsules whole and not to crush or open the capsules.
| ERIVEDGE® [vismodegib] capsule |
|
| Manufactured by: | |
| Patheon, Inc. | |
| Mississauga, Canada | ERIVEDGE is a registered trademark of Genentech, Inc. |
| ©2012 Genentech, Inc. | |
| Distributed by: | |
| Genentech USA, Inc. | |
| A Member of the Roche Group | |
| 1 DNA Way | |
| South San Francisco, CA 94080-4990 | |
MEDICATION GUIDE
ERIVEDGE
®
(EH-rih-vej)
(vismodegib)
capsule
Read this Medication Guide before you start taking ERIVEDGE and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about ERIVEDGE?
ERIVEDGE can cause your baby to die before it is born (be stillborn) or cause your baby to have severe birth defects.
For females who can become pregnant:
- You should talk with your healthcare provider about the risks of ERIVEDGE to your unborn child.
- Your healthcare provider should do a pregnancy test within 7 days before you start taking ERIVEDGE to find out if you are pregnant.
- In order to avoid pregnancy, you should start using highly effective birth control before you start ERIVEDGE, and continue to use highly effective birth control during treatment, and for 7 months after your last dose of ERIVEDGE. Talk with your healthcare provider about what birth control method is right for you during this time.
- Talk to your healthcare provider right away if you have unprotected sex or if you think that your birth control has failed.
- Tell your healthcare provider right away if you become pregnant or think that you may be pregnant.
For males:
- You should always use a condom with a spermicide, even if you have had a vasectomy, during sex with female partners while you are taking ERIVEDGE and for 2 months after your last dose to protect your female partner from being exposed to ERIVEDGE.
- Tell your healthcare provider right away if your partner becomes pregnant or thinks she is pregnant while you are taking ERIVEDGE.
Exposure to ERIVEDGE during pregnancy:
If you think that you or your female partner may have been exposed to ERIVEDGE during pregnancy, talk to your healthcare provider right away. Pregnant women are encouraged to participate in a program that collects information about exposure to ERIVEDGE during pregnancy, and the effects on the mother and her unborn child. This program is called the ERIVEDGE pregnancy pharmacovigilance program. You may participate in this program by calling the Genentech Adverse Event Line at 1-888-835-2555.
What is ERIVEDGE?
ERIVEDGE is a prescription medicine used to treat adults with a type of skin cancer, called basal cell carcinoma, that has spread to other parts of the body or that has come back after surgery or that your healthcare provider decides cannot be treated with surgery or radiation.
It is not known if ERIVEDGE is safe and effective in children.
What should I tell my healthcare provider before taking ERIVEDGE?
Before taking ERIVEDGE, tell your healthcare provider if you:
- are pregnant or plan to become pregnant. See "What is the most important information I should know about ERIVEDGE?"
- are breastfeeding or plan to breastfeed. It is not known if ERIVEDGE passes into your breast milk. You and your healthcare provider should decide if you will take ERIVEDGE or breastfeed. You should not do both.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take ERIVEDGE?
- Take ERIVEDGE exactly as your healthcare provider tells you.
- You can take ERIVEDGE with or without food.
- Swallow ERIVEDGE capsules whole. Do not open or crush the capsules.
- Take ERIVEDGE one time each day.
- If you miss a dose, skip the missed dose. Just take your next scheduled dose.
What should I avoid while taking ERIVEDGE?
- Do not donate blood or blood products while you are taking ERIVEDGE and for 7 months after your last dose.
What are the possible side effects of ERIVEDGE?
ERIVEDGE can cause serious side effects, including:
The most common side effects of ERIVEDGE are:
- muscle spasms
- hair loss
- change in how things taste or loss of taste
- weight loss
- tiredness
- nausea
- diarrhea
- decreased appetite
- constipation
- vomiting
- joint aches
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of ERIVEDGE. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Genentech, Inc. at 1-888-835-2555.
How should I store ERIVEDGE?
- Store ERIVEDGE at room temperature between 68°F to 77°F (20°C to 25°C).
Keep ERIVEDGE and all medicines out of the reach of children.
General information about ERIVEDGE
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ERIVEDGE for a condition for which it was not prescribed. Do not give ERIVEDGE to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about ERIVEDGE. If you would like more information, ask your health care provider. You can ask your healthcare provider or pharmacist for the FDA-approved information about ERIVEDGE that is written for healthcare professionals.
For more information, call 1-855-737-4833 or visit www.erivedge.com
What are the ingredients in ERIVEDGE?
Active ingredient: vismodegib
Inactive ingredients: microcrystalline cellulose, lactose monohydrate, sodium lauryl sulfate, povidone, sodium starch glycolate, talc, magnesium stearate (non bovine). The capsule shell contains gelatin, titanium dioxide, red iron oxide, and black iron oxide. The black printing ink contains shellac and black iron oxide.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
MG Issued: 01/2012
Manufactured by:
Patheon, Inc.
Mississauga, Canada
Distributed by:
Genentech USA, Inc.
A Member of the Roche Group
1 DNA Way
South San Francisco, CA 940804990
ERIVEDGE is a registered trademark of Genentech, Inc.
©2012 Genentech, Inc.
Representative sample of labeling (see the HOW SUPPLIED section for complete listing):
PRINCIPAL DISPLAY PANEL - 150 mg Capsule Bottle Label
NDC 50242-140-01
Erivedge®
(vismodegib)
capsules
150 mg
Each capsule contains 150 mg of
vismodegib
Attention Pharmacist: Dispense
the accompanying Medication
Guide to each patient.
28 capsules
Rx only
Genentech
10138542

ERIVEDGEvismodegib CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||