Epivir
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- EPIVIR DESCRIPTION
- MICROBIOLOGY
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- EPIVIR CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- EPIVIR ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- SPL PATIENT PACKAGE INSERT
- INACTIVE INGREDIENT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
EPIVIR DESCRIPTION
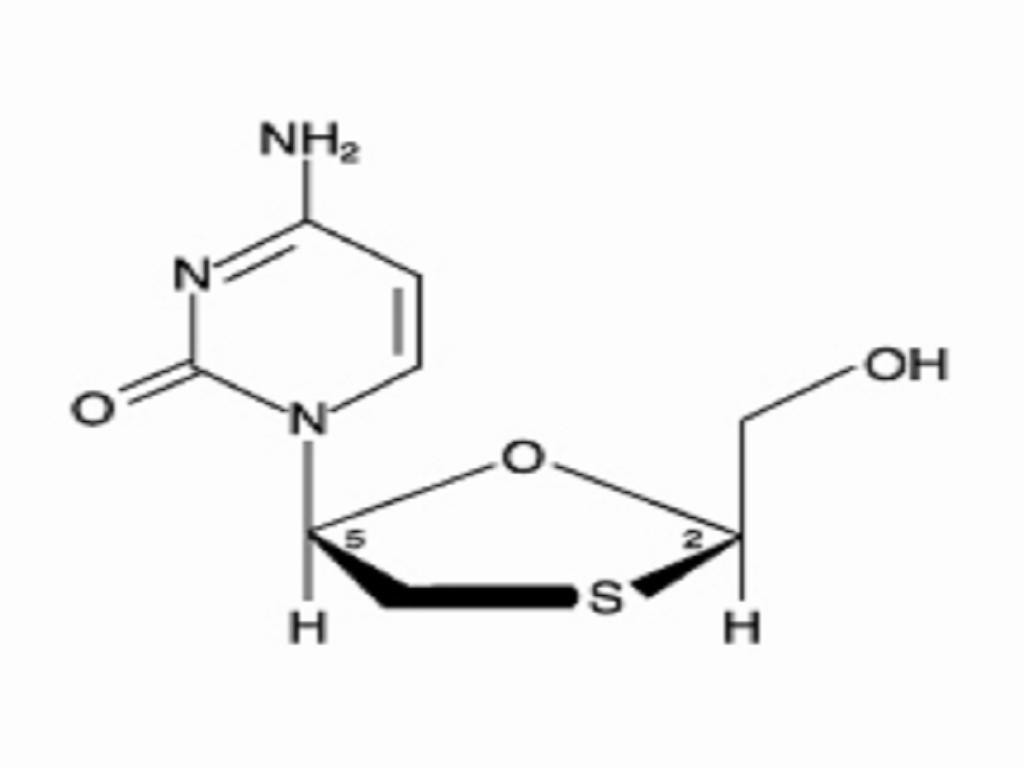
MICROBIOLOGY
Mechanism of Action:Antiviral Activity:
Resistance:
Cross-Resistance:
CLINICAL PHARMACOLOGY
Pharmacokinetics in Adults:Special Populations:
++
Drug Interactions:
INDICATIONS & USAGE
-
● Due to high rates of resistance development in treated patients, initiation of lamivudine treatment should only be considered when the use of an alternative antiviral agent with a higher genetic barrier to resistance is not available or appropriate.
-
●
-
● Description of Clinical Studies:
-
● Study 3 was a randomized, partially-blind, 3-arm study conducted primarily in North America and Europe in patients who had ongoing evidence of active chronic hepatitis B despite previous treatment with interferon alfa. The study compared EPIVIR-HBV 100 mg once daily for 52 weeks, followed by either EPIVIR-HBV 100 mg or matching placebo once daily for 16 weeks (Arm 1), versus placebo once daily for 68 weeks (Arm 2). (A third arm using a combination of interferon and lamivudine is not presented here because there was not sufficient information to evaluate this regimen.)
-
● Principal endpoint comparisons for the histologic and serologic outcomes in lamivudine (100 mg daily) and placebo recipients in placebo-controlled studies are shown in the following tables.
-
●
EPIVIR CONTRAINDICATIONS
WARNINGS
Lactic Acidosis/Severe Hepatomegaly With Steatosis:Important Differences Between Lamivudine-Containing Products, HIV Testing, and Risk of Emergence of Resistant HIV:
Posttreatment Exacerbations of Hepatitis:
Pancreatitis:
PRECAUTIONS
General:Emergence of Resistance-Associated HBV Mutations:
Limitations of Populations Studied:
Assessing Patients During Treatment:
Patients With Impaired Renal Function:
INFORMATION FOR PATIENTS
DRUG INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
EPIVIR ADVERSE REACTIONS
Clinical Trials In Chronic Hepatitis B:
Lamivudine in Patients With HIV:
Pediatric Patients With Hepatitis B:
Pediatric Patients With HIV Infection:
Observed During Clinical Practice:
OVERDOSAGE
DOSAGE & ADMINISTRATION
Adults:Pediatric Patients:
Dose Adjustment:
HOW SUPPLIED
REFERENCES
SPL PATIENT PACKAGE INSERT
-
● You have HIV infection.
-
● You are pregnant or if you become pregnant while taking EPIVIR-HBV.
-
● You are breastfeeding.
-
● You have diabetes. Each 20-mL dose (100 mg) of EPIVIR-HBV Oral Solution contains 4 grams of sucrose.
-
● Also talk to your doctor or healthcare provider about:
-
● Problems with your blood counts.
-
● Problems with your kidneys.
-
● Problems with your pancreas.
-
● Any side effects or unusual symptoms during treatment.
-
● How should I store EPIVIR-HBV Tablets and Oral Solution?
-
● EPIVIR-HBV Tablets and Oral Solution should be stored at room temperature. They do not require refrigeration. Keep EPIVIR-HBV and all medicines out of the reach of children.
INACTIVE INGREDIENT
HYPROMELLOSESMAGNESIUM STEARATE
CELLULOSE, MICROCRYSTALLINE
POLYETHYLENE GLYCOL
POLYSORBATE 80
SODIUM STARCH GLYCOLATE TYPE A POTATO
TITANIUM DIOXIDE
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

EpivirLamivudine TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!