enema mineral oil
Major Pharmaceuticals Enema Mineral Oil Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (in each 118 mL delivered dose)
- Purpose
- Use
- Warnings
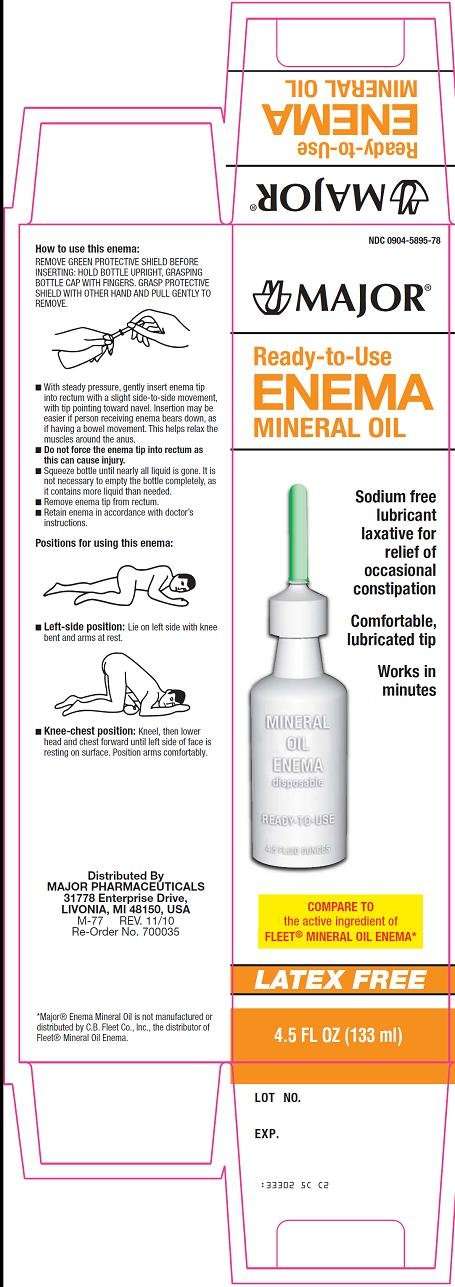
- Directions
- enema mineral oil Other information
- Questions or comments?
- Package/Label Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient (in each 118 mL delivered dose)
Mineral oil 100%
Purpose
Lubricant laxative
Use
- for relief of occasional constipation
Warnings
For rectal use only
Ask a doctor before use if you have
- abdominal pain, nausea or vomiting
- notice a sudden change in bowel habits that persists over a period of 2 weeks
Stop use and ask a doctor if
- you have rectal bleeding or failure to have a bowel movement after use of a laxative because this may indicate a serious condition
- you need to use a laxative for more than one week
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Directions
Single daily dose
|
adults & children 12 years and over |
1 bottle |
|
children 2 to under 12 years |
one-half bottle |
|
children under 2 years |
DO NOT USE |
Other information
- store at 20º-25ºC (68º-77ºF)
- this product generally produces a bowel movement in 2-15 minutes
Questions or comments?
1-800-616-2471
Package/Label Principal Display Panel
Ready-to-Use
ENEMA MINERAL OIL
Sodium free lubricant laxative for relief of occasional constipation
Comfortable, lubricated tip
Works in minutes
COMPARE TO the active ingredient of FLEET® MINERAL OIL ENEMA
LATEX FREE
Enema Mineral Oil Carton Image 1
Enema Mineral Oil Carton Image 2
enema mineral oilMineral Oil LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!