ELFMineral Blemish Kit Box
Hangzhou Facecare Cosmetics Co., Ltd.
Hangzhou Facecare Cosmetics Co., Ltd.
Drug Fact
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredient:
Sulfur 3.0%
Purpose
Purpose:
Anti Acne
Uses
Uses:
Heal and disguise blemishes with the all natural Mineral Blemish Kit. This translucent powder has active acne fighting ingredients like nature sulfur to treat acne and prevent future breakouts while penetrate deep into the pores to control blackheads.
Warning:
For external use only
When Using This Product:
Do not get into eyes
Avoid on wounds and on areas of eczema
Stop Use and Ask A Doctor If:
Rash or irritation develops and lasts
Keep Out of Reach of Children:
If swallowed get medical help or contact a Poison Control Center immediately
Directions:
Take the concealer brush and dap into powder. Apply formula on areas that need spot treatment. Use nightly or daily to treat and prevent future breakouts.
Other Information:
Store at room temperature 20-25C (68-77F)
Inactive Ingredient:
Zince Stearate, Barium Sulfate, Silica, Kaolin, Dimethicone/Vinyl Dimethicone Crosspolymer, Corn Starch Modified, Alba (Willow) Bark Extract, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Dextrin, Magnesium Myristate, Calcium Sodium Phosphosilicate, Mica, Bismuth Oxychloride, Titanium Dioxide
May contain:
Iron Oxides (CI 77491, CI 77492, CI 77499)
labe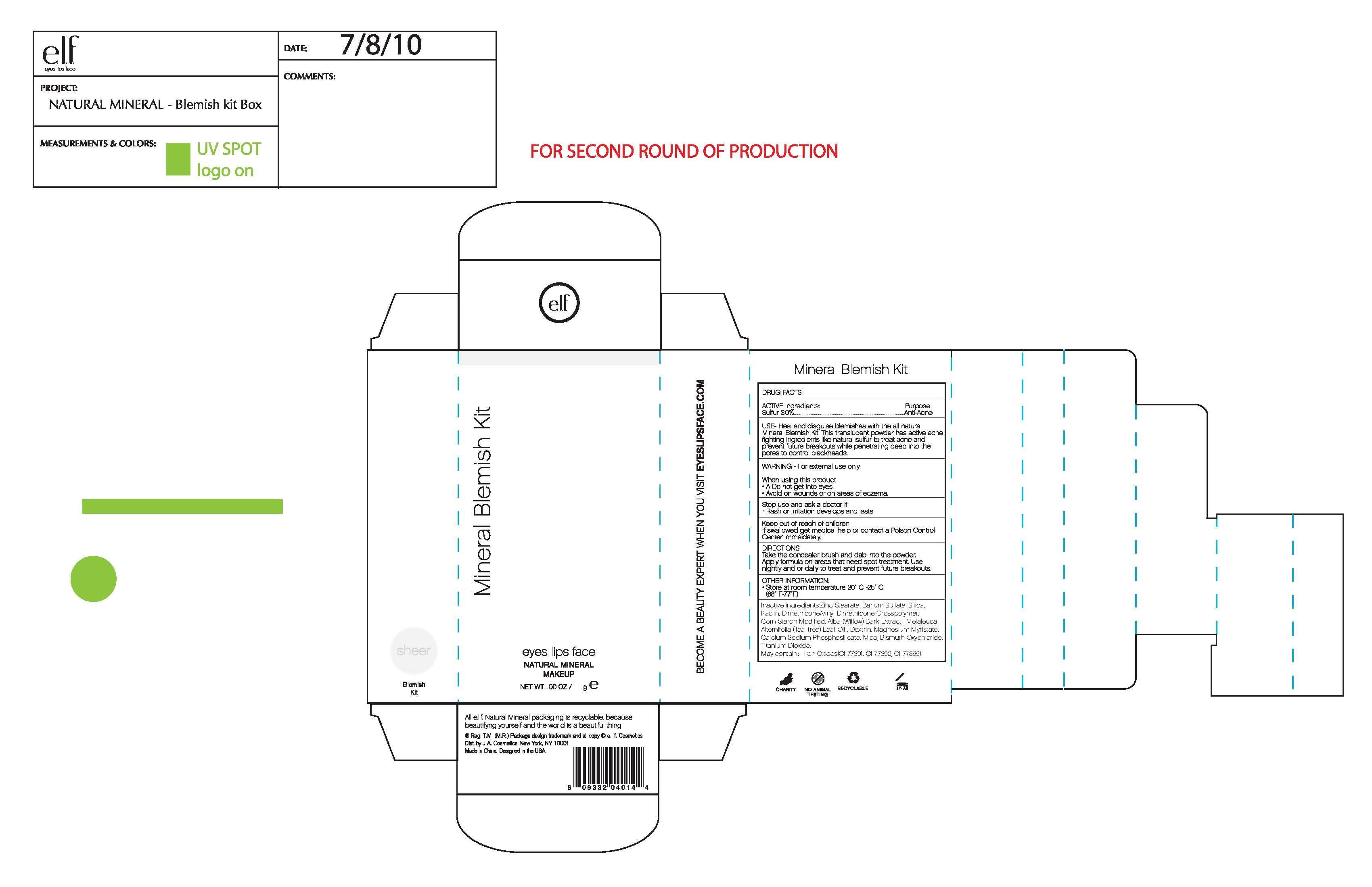
ELFMineral Blemish Kit BoxSULFUR POWDER
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||