DOXOrubicin Hydrochloride
Sun Pharmaceutical Industries Limited
DOXOrubicin Hydrochloride Injection, USPFor Intravenous Use Only
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNINGS
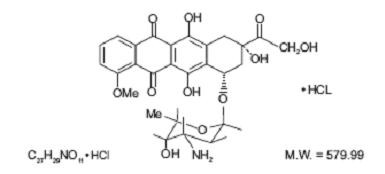
- DOXORUBICIN HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- DOXORUBICIN HYDROCHLORIDE INDICATIONS AND USAGE
- DOXORUBICIN HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DOXORUBICIN HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOXORUBICIN HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- PATIENT INFORMATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL 25ML
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - CARTON 25ML
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL 100ML
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - CARTON 100ML
FULL PRESCRIBING INFORMATION
WARNINGS
- Severe local tissue necrosis will occur if there is extravasation during administration (see DOSAGE AND ADMINISTRATION). Doxorubicin must not be given by the intramuscular or subcutaneous route.
- Myocardial toxicity manifested in its most severe form by potentially fatal congestive heart failure (CHF) may occur either during therapy or months to years after termination of therapy. The probability of developing impaired myocardial function based on a combined index of signs, symptoms, and decline in left ventricular ejection fraction (LVEF) is estimated to be 1 to 2% at a total cumulative dose of 300 mg/m2 of doxorubicin, 3 to 5% at a dose of 400 mg/m2, 5 to 8% at 450 mg/m2, and 6 to 20% at 500 mg/m2. The risk of developing CHF increases rapidly with increasing total cumulative doses of doxorubicin in excess of 400 mg/m2. Risk factors (active or dormant cardiovascular disease, prior or concomitant radiotherapy to the mediastinal/pericardial area, previous therapy with other anthracyclines or anthracenediones, concomitant use of other cardiotoxic drugs) may increase the risk of cardiac toxicity. Cardiac toxicity with doxorubicin may occur at lower cumulative doses whether or not cardiac risk factors are present. Pediatric patients are at increased risk for developing delayed cardiotoxicity.
- Secondary acute myelogenous leukemia (AML) or myelodysplastic syndrome (MDS) have been reported in patients treated with anthracyclines, including doxorubicin (see ADVERSE REACTIONS). The occurrence of refractory secondary AML or MDS is more common when anthracyclines are given in combination with DNA-damaging anti-neoplastic agents or radiotherapy, when patients have been heavily pretreated with cytotoxic drugs, or when doses of anthracyclines have been escalated. The rate of developing secondary AML or MDS has been estimated in an analysis of 8563 patients with early breast cancer treated in 6 studies conducted by the National Surgical Adjuvant Breast and Bowel Project (NSABP), including NSABP B-15. Patients in these studies received standard doses of doxorubicin and standard or escalated doses of cyclophosphamide (AC) adjuvant chemotherapy and were followed for 61,810 patient years. Among 4483 such patients who received conventional doses of AC, 11 cases of AML or MDS were identified, for an incidence of 0.32 cases per 1000 patient years (95% CI, 0.16 to 0.57) and a cumulative incidence at 5 years of 0.21% (95% CI, 0.11 to 0.41%). In another analysis of 1474 patients with breast cancer who received adjuvant treatment with doxorubicin-containing regimens in clinical trials conducted at University of Texas M.D. Anderson Cancer Center, the incidence was estimated at 1.5% at 10 years. In both experiences, patients who received regimens with higher cyclophosphamide dosages, who received radiotherapy, or who were aged 50 or older had an increased risk of secondary AML or MDS. Pediatric patients are also at risk of developing secondary AML.
- Dosage should be reduced in patients with impaired hepatic function.
- Severe myelosuppression may occur.
- Doxorubicin should be administered only under the supervision of a physician who is experienced in the use of cancer chemotherapeutic agents.
DOXORUBICIN HYDROCHLORIDE DESCRIPTION
Streptomyces peucetius caesius
CLINICAL PHARMACOLOGY
Pharmacokinetics
2
Distribution. 2
22
Metabolism.
Excretion. 2
Pharmacokinetics in Special Populations
Pediatric. 2222
Geriatric PRECAUTIONS, Geriatric Use .
Gender22
Race.
Hepatic Impairment DOSAGE AND ADMINISTRATION
Renal Impairment.
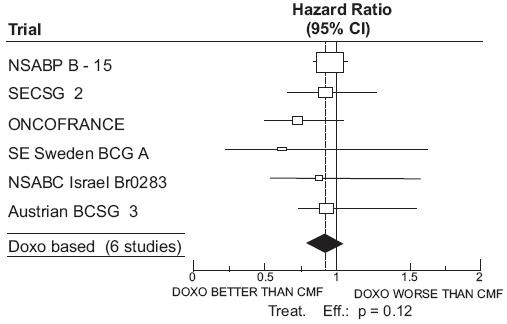
CLINICAL STUDIES
| Study (starting year) |
Regimens | No. of Cycles | No. of Patients | Doxorubicin-Containing Regimens vs CMF HR (95% CI) |
|
|---|---|---|---|---|---|
| DFS | OS | ||||
|
Abbreviations: DFS = disease free survival; OS = overall survival; AC = doxorubicin, cyclophosphamide; AVbCMF = doxorubicin, vinblastine, cyclophosphamide, methotrexate, 5-fluorouracil; CMF = cyclophosphamide, methotrexate, 5-fluorouracil; CMFVA = cyclophosphamide, methotrexate, 5-fluorouracil, vincristine, doxorubicin; FAC = 5-fluorouracil, doxorubicin, cyclophosphamide; FACV = 5-fluorouracil, doxorubicin, cyclophosphamide, vincristine; HR = hazard ratio; CI = confidence interval |
|||||
| NSABP B-15 (1984) |
AC CMF |
4 6 |
1562 776 |
0.93 (0.82 to 1.06) |
0.97 (0.83 to 1.12) |
| SECSG 2 (1976) |
FAC CMF |
6 6 |
260 268 |
0.86 (0.66 to 1.13) |
0.93 (0.69 to 1.26) |
| ONCOFRANCE (1978) |
FACV CMF |
12 12 |
138 113 |
0.71 (0.49 to 1.03) |
0.65 (0.44 to 0.96) |
| SE Sweden BCG A (1980) |
AC CMF |
6 6 |
21 22 |
0.59 (0.22 to 1.61) |
0.53 (0.21 to 1.37) |
| NSABC Israel Br0283 (1983) |
AVb CMF CMF |
4 6 6 |
55 50 |
0.91 (0.53 to 1.57) |
0.88 (0.47 to 1.63) |
| Austrian BCSG 3 (1984) |
CMFVA CMF |
6 8 |
121 124 |
1.07 (0.73 to 1.55) |
0.93 (0.64 to 1.35) |
| Combined Studies
|
Doxorubicin-Containing Regimens CMF |
2157 1353 |
0.91 (0.82 to 1.01) |
0.91 (0.81 to 1.03) |
|
Figure 1. Meta-analysis of Disease-Free Survival
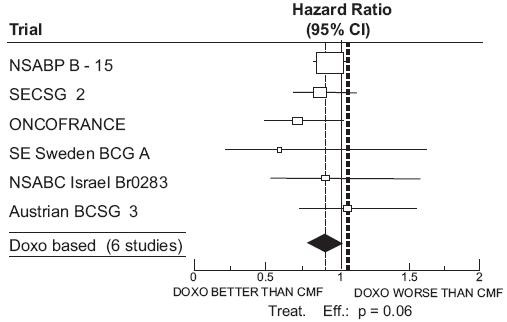
Figure 2. Meta-analysis of Overall Survival
DOXORUBICIN HYDROCHLORIDE INDICATIONS AND USAGE
DOXORUBICIN HYDROCHLORIDE CONTRAINDICATIONS
3 WARNINGS DOSAGE AND ADMINISTRATION.
WARNINGS
General
Doxorubicin should be administered only under the supervision of qualified physicians experienced in the use of cytotoxic therapy. Patients should recover from acute toxicities of prior cytotoxic treatment (such as stomatitis, neutropenia, thrombocytopenia, and generalized infections) before beginning treatment with doxorubicin. Also, initial treatment with doxorubicin should be preceded by a careful baseline assessment of blood counts; serum levels of total bilirubin, AST, and creatinine; and cardiac function as measured by left ventricular ejection function (LVEF). Patients should be carefully monitored during treatment for possible clinical complications due to myelosuppression. Supportive care may be necessary for the treatment of severe neutropenia and severe infectious complications. Monitoring for potential cardiotoxicity is also important, especially with greater cumulative exposure to doxorubicin. Doxorubicin may potentiate the toxicity of other anticancer therapies (see PRECAUTIONS, Drug Interactions ).
Cardiac Function
222222222222
PRECAUTIONS, General DOSAGE AND ADMINISTRATION
Monitoring Cardiac Function
Hematologic Toxicity
Secondary Leukemia
Effects at Site of Injection
DOSAGE AND ADMINISTRATION, Instruction for Use/Handling
Extravasation
DOSAGE AND ADMINISTRATION
Hepatic Impairment
DOSAGE AND ADMINISTRATION
Immunosuppressant Effects/Increased Susceptibility to Infections
Pregnancy Category D
PRECAUTIONS
General
Doxorubicin is not an anti-microbial agent. Doxorubicin is emetigenic. Antiemetics may reduce nausea and vomiting; prophylactic use of antiemetics should be considered before administration of doxorubicin, particularly when given in conjunction with other emetigenic drugs. Doxorubicin should not be administered in combination with other cardiotoxic agents unless the patient’s cardiac function is closely monitored. Patients receiving doxorubicin after stopping treatment with other cardiotoxic agents, especially those with long half-lives such as trastuzumab, may also be at an increased risk of developing cardiotoxicity. Physicians should avoid doxorubicin-based therapy for up to 24 weeks after stopping trastuzumab when possible. If doxorubicin used before this time, careful monitoring of cardiac function is recommended (See WARNINGS , DOSAGE AND ADMINISTRATION ).
Information for Patients
Patients should be informed of the expected adverse effects of doxorubicin, including gastrointestinal symptoms (nausea, vomiting, diarrhea, and stomatitis) and potential neutropenic complications. Patients should consult their physician if vomiting, dehydration, fever, evidence of infection, symptoms of CHF, or injection-site pain occurs following therapy with doxorubicin. Patients should be informed that they will almost certainly develop alopecia. Patients should be advised that their urine may appear red for 1 to 2 days after administration of doxorubicin and that they should not be alarmed. Patients should understand that there is a risk of irreversible myocardial damage associated with treatment with doxorubicin, as well as a risk of treatment-related leukemia. Because doxorubicin may induce chromosomal damage in sperm, men undergoing treatment with doxorubicin should use effective contraceptive methods. Women treated with doxorubicin may develop irreversible amenorrhea, or premature menopause.
Drug Interactions
Paclitaxel:
Progesterone: 2
Verapamil:
Cyclosporine:
Dexrazoxane: 22
Cytarabine:
Sorafenib: .
Cyclophosphamide:
Literature reports have also described the following drug interactions: ®WARNINGS
Laboratory Tests
WARNINGS
Carcinogenesis, Mutagenesis, and Impairment of Fertility
WARNINGS in vitroin vitroin vivo
WARNINGS, Hematologic Toxicity
Pregnancy Category D
WARNINGS
Nursing Mothers
CLINICAL PHARMACOLOGY, Pharmacokinetics).
Pediatric Use
WARNINGS
Geriatric Use
DOXORUBICIN HYDROCHLORIDE ADVERSE REACTIONS
Cardiotoxicity WARNINGS
Cutaneous
Gastrointestinal
Hematologic WARNINGS.
Hypersensitivity
Neurological
Ocular
Other --
Adverse Reactions in Patients with Early Breast Cancer Receiving Doxorubicin-Containing Adjuvant Therapy
AC |
Conventional CMF | |
|---|---|---|
| N=1492 | N=739 | |
| Treatment administration
|
||
| Mean number of cycles |
3.8
|
5.5
|
| Total cycles |
5676 |
4068 |
| Adverse events, % of patients |
||
| Leukopenia |
||
| Grade 3 (1,000 to 1,999/mm3) |
3.4 |
9.4 |
| Grade 4 (<1000/mm3) |
0.3 |
0.3 |
| Thrombocytopenia |
||
| Grade 3 (25,000 to 49,999/mm3) |
0 |
0.3 |
| Grade 4 (<25,000/mm3) |
0.1 |
0 |
| Shock, sepsis |
1.5 |
0.9 |
| Systemic infection |
2.4 |
1.2 |
| Nausea and vomiting |
||
| Nausea only |
15.5 |
42.8 |
| Vomiting ≤12 hours |
34.4 |
25.2 |
| Vomiting >12 hours |
36.8 |
12 |
| Intractable |
4.7 |
1.6 |
| Alopecia |
92.4 |
71.4 |
| Partial |
22.9 |
56.3 |
| Complete |
69.5 |
15.1 |
| Weight loss |
||
| 5 to 10% |
6.2 |
5.7 |
| >10% |
2.4 |
2.8 |
| Weight gain |
||
| 5 to 10% |
10.6 |
27.9 |
| >10% |
3.8 |
14.3 |
| Cardiac function |
||
| Asymptomatic |
0.2 |
0.1 |
| Transient |
0.1 |
0 |
| Symptomatic |
0.1 |
0 |
| Treatment-related death |
0 |
0 |
OVERDOSAGE
Acute overdosage with doxorubicin enhances the toxic effect of mucositis, leukopenia, and thrombocytopenia. Treatment of acute overdosage consists of treatment of the severely myelosuppressed patient with hospitalization, antimicrobials, platelet transfusions, and symptomatic treatment of mucositis. Use of hemopoietic growth factor (G-CSF, GM-CSF) may be considered. The 100 mL (2 mg/mL) doxorubicin hydrochloride injection vials are packaged as multiple dose vials and caution should be exercised to prevent inadvertent overdosage. Cumulative dosage with doxorubicin increases the risk of cardiomyopathy and resultant congestive heart failure (see WARNINGS ). Treatment consists of vigorous management of congestive heart failure with digitalis preparations, diuretics, and after-load reducers such as ACE inhibitors.
DOXORUBICIN HYDROCHLORIDE DOSAGE AND ADMINISTRATION
WARNINGS PRECAUTIONS, General
WARNINGS
2
2
CLINICAL PHARMACOLOGY, Clinical Studies ADVERSE REACTIONS, Adverse Reactions in Patients with Early Breast Cancer Receiving Doxorubicin-Containing Adjuvant Therapy 22
Dose Modifications
33
|
Plasma bilirubin concentration (mg/dL)
|
Dosage reduction (%)
|
| 1.2 to 3 |
50 |
| 3.1 to 5
|
75 |
Directions for Use
®
Handling and Disposal
1 to 4
- Personnel should be trained in good technique for handling.
- Pregnant staff should be excluded from working with this drug.
- Personnel handling doxorubicin should wear protective clothing: goggles, gowns, and disposable gloves and masks.
- All items used for administration, or cleaning, including gloves, should be placed in high-risk waste-disposal bags for high-temperature incineration.
- Spillage or leakage should be treated with dilute sodium hypochlorite (1% available chlorine) solution, preferably by soaking, and then water.
- All cleaning materials should be disposed of as indicated previously.
- In case of skin contact, thoroughly wash the affected area with soap and water or sodium bicarbonate solution. However, do not abrade the skin by using a scrub brush.
- In case of contact with the eye(s), hold back the eyelid(s) and flush the affected eye(s) with copious amounts of water for at least 15 minutes. Then seek medical evaluation by a physician.
- Always wash hands after removing gloves.
HOW SUPPLIED
NDC 62756-826-40:
Protect from light.
NDC 62756-827-40:
Protect from light.
REFERENCES
- NIOSH Alert: Preventing occupational exposures to antineoplastic and other hazardous drugs in healthcare settings. 2004. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2004-165.
- OSHA Technical Manual, TED 1-0.15A, Section VI: Chapter 2. Controlling Occupational Exposure to Hazardous Drugs. OSHA, 1999. http://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2.html
- American Society of Health-System Pharmacists. ASHP guidelines on handling hazardous drugs. Am J Health-Syst Pharm. 2006; 63:1172-1193.
- Polovich, M., White, J. M., & Kelleher, L.O. (eds.) 2005. Chemotherapy and biotherapy guidelines and recommendations for practice (2nd. ed.) Pittsburgh , PA : Oncology Nursing Society.
PATIENT INFORMATION
DOXOrubicin Hydrochloride Injection, USP
What is the most important information I should know about doxorubicin?
Doxorubicin may cause serious side effects including:
-
Heart problems. Doxorubicin may cause heart problems that may lead to death. These problems can happen during your treatment or months to years after stopping treatment. In some cases heart problems are irreversible. Your chance of heart problems is higher if you:
- already have heart problems
- have a history of radiation therapy or are currently receiving radiation therapy to your chest
- have had treatment with certain other anti-cancer medicines
- take other medicines that can affect your heart
- shortness of breath
- swelling of your feet and ankles
- cough
- fast heartbeat
Your doctor should do tests to check your heart before, during, and after your treatment with doxorubicin.
- Secondary cancers. Some people who have received doxorubicin have developed acute myelogenous leukemia (AML) or myelodysplastic syndrome (MDS). Your chance of developing a secondary cancer is higher if you receive doxorubicin along with other anti-cancer medicines or with radiation therapy.
- Decreased blood cell counts. Doxorubicin can cause a severe decrease in neutrophils (a type of white blood cells important in fighting in bacterial infections), red blood cells (blood cells that carry oxygen to the tissues), and platelets (important for clotting and to control bleeding).Your doctor will check your blood cell count during your treatment with doxorubicin and after you have stopped your treatment.
What is doxorubicin?
Who should not receive doxorubicin?
Do not receive doxorubicin if:
- your blood cell counts are too low: platelets (which help your blood to clot), red blood cells (which help to carry iron and oxygen throughout your body), and white blood cells (which help to fight infection)
- you have a severe liver problem
- you have had a recent heart attack or have severe heart problems
- you have had previous treatment with doxorubicin or certain other anticancer medicines and received the maximum dose allowed
- you are allergic to certain other anti-cancer medicines, doxorubicin hydrochloride, or any other ingredient in doxorubicin hydrochloride injection. See the end of this leaflet for a complete list of ingredients in doxorubicin hydrochloride injection.
What should I tell my doctor before receiving doxorubicin?
- have heart problems
- have had radiation treatment or currently receiving radiation therapy
- are over the age of 50
- have liver problems
- plan to receive any vaccines. Talk to your doctor about which vaccines are safe for you to receive during your treatment with doxorubicin. See “What should I avoid while receiving doxorubicin?”
- have any other medical conditions
- are pregnant or plan to become pregnant. Doxorubicin can harm your unborn baby. Women who may become pregnant should use effective birth control (contraception). Talk to your doctor about the best way to prevent pregnancy while receiving doxorubicin.
- are breastfeeding or plan to breast feed. Doxorubicin can pass into your breast milk and harm your baby. You and your doctor should decide if you will receive doxorubicin or breastfeed. You should not do both.
Tell your doctor about all the medicines you take,
How will I receive doxorubicin?
- Your doctor will prescribe doxorubicin in an amount that is right for you.
- Doxorubicin will be given to you by intravenous (IV) infusion into your vein.
- Your doctor will do regular blood tests to check for side effects of doxorubicin.
- Before receiving doxorubicin you may receive other medicines to prevent or treat side effects.
- Caregivers of children receiving doxorubicin should take precautions (such as wearing latex gloves) to prevent contact with the patient’s urine and other body fluids for at least 5 days after each treatment.
What should I avoid while taking doxorubicin?
- Avoid receiving live vaccines during treatment with doxorubicin. Talk to your doctor to find out which vaccines are safe for you while receiving doxorubicin. See “What should I tell my doctor before receiving doxorubicin?”
What are the possible side effects of doxorubicin?
Doxorubicin can cause serious side effects including:
- See “What is the most important information I should know about doxorubicin?”
Infusion site reactions.
- pain at injection site
- skin redness or swelling
- burning or stinging
- open skin sores at injection site
Change in the color of your urine.
Infection.
- fever (temperature of 100.4 F or greater) chills or shivering
- cough that brings up mucus
- burning or pain with urination
Doxorubicin may cause lower sperm counts and sperm problems in men.
Irreversible amenorrhea or early menopause.
- hair loss (alopecia). Your hair may re-grow after your treatment.
- darkening of your nails or separation of your nails from your nailbed
- nausea
- vomiting
- lack of appetite or increased thirst
- bruise or bleed more easily
- abnormal heart beat
- a secondary cancer may occur when doxorubicin is combined with other chemotherapy agents.
- mouth sores
- weight changes
- stomach (abdominal) pain
- diarrhea
- eye problems
- allergic reactions. Call your doctor right away if you have any of the following symptoms of an allergic reaction
- rash
- fever
- flushed face
- dizziness or feel faint
- hives
- shortness of breath or trouble breathing
- itching
- swelling of your lips or tongue
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of doxorubicin.
What are the ingredients of doxorubicin hydrochloride injection?
Caraco Pharmaceutical Laboratories, Ltd.
Sun Pharmaceutical Industries Ltd.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL 25ML
NDC 62756-826-40
DOXOrubicin Hydrochloride Injection, USP
50 mg/25 mL (2 mg/mL)
FOR INTRAVENOUS USE ONLY
STERILE ISOTONIC SOLUTION
CAUTION: CYTOTOXIC AGENT
Rx only
25 mL SINGLE DOSE VIAL
SUN PHARMA

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - CARTON 25ML
NDC 62756-826-40
DOXOrubicin Hydrochloride Injection, USP
50 mg/25 mL
(2 mg/mL)
FOR INTRAVENOUS USE ONLY
STERILE ISOTONIC SOLUTION
CYTOTOXIC AGENT
Rx only
25 mL SINGLE DOSE VIAL
SUN PHARMA
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL 100ML
NDC 62756-827-40
DOXOrubicin Hydrochloride Injection, USP
200 mg/100 mL (2 mg/mL)
FOR INTRAVENOUS USE ONLY
STERILE ISOTONIC SOLUTION
CAUTION: CYTOTOXIC AGENT
MULTIPLE DOSE VIAL
Rx only
100 mL MULTI-DOSE VIAL
SUN PHARMA

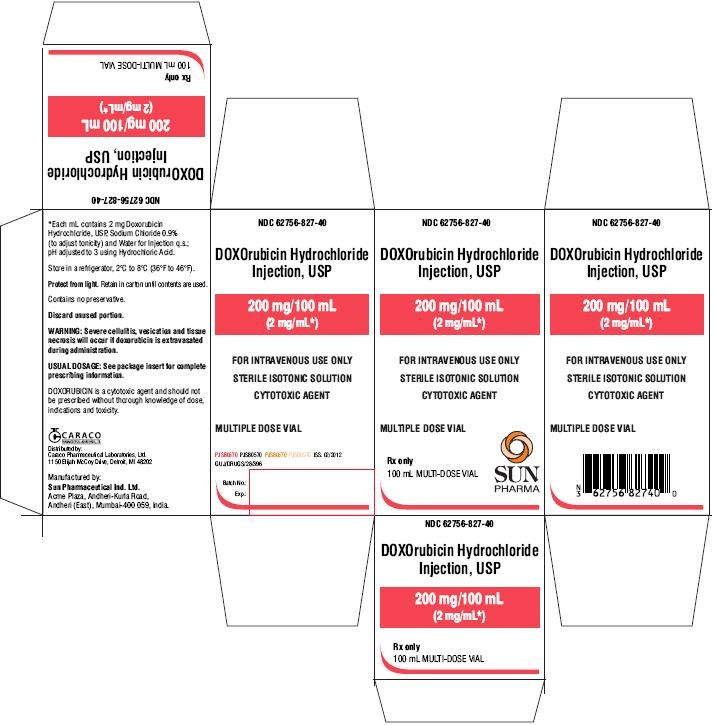
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - CARTON 100ML
NDC 62756-827-40
DOXOrubicin Hydrochloride Injection, USP
200 mg/100 mL
(2 mg/mL)
FOR INTRAVENOUS USE ONLY
STERILE ISOTONIC SOLUTION
CYTOTOXIC AGENT
MULTIPLE DOSE VIAL
Rx only
100 mL MULTI-DOSE VIAL
SUN PHARMA
DOXOrubicin HydrochlorideDOXOrubicin Hydrochloride INJECTION, SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DOXOrubicin HydrochlorideDOXOrubicin Hydrochloride INJECTION, SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||