DOCUSATE SODIUM
Docusate Sodium capsules USP 100mg
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (in each capsule)
- Purpose
- DOCUSATE SODIUM Uses
- Warnings
- Directions
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient (in each capsule)
Docusate sodium 100 mg
Purpose
Stool softener
DOCUSATE SODIUM Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 12 to 72 hours
Warnings
Do not use
- if you are presently taking mineral oil, unless told to do so by a doctor
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
Stop use and ask a doctor if
- you have rectal bleeding or fail to have a bowel movement after use of a laxative. These could be signs of a serious condition.
- you need to use a laxative for more than 1 week
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- Take only by mouth. Doses may be taken as a single daily dose or in divided doses.
| adults and children 12 years and over | take 1-3 capsules daily |
| children 2 to under 12 years of age | take 1 capsule daily |
| children under 2 years | ask a doctor |
Inactive ingredients
D&C Red No. 33, FD&C Red No. 40, FD&C Yellow No. 6, gelatin, glycerin, methylparaben, PEG 400, propylene glycol, propylparaben, sorbitol, titanium dioxide
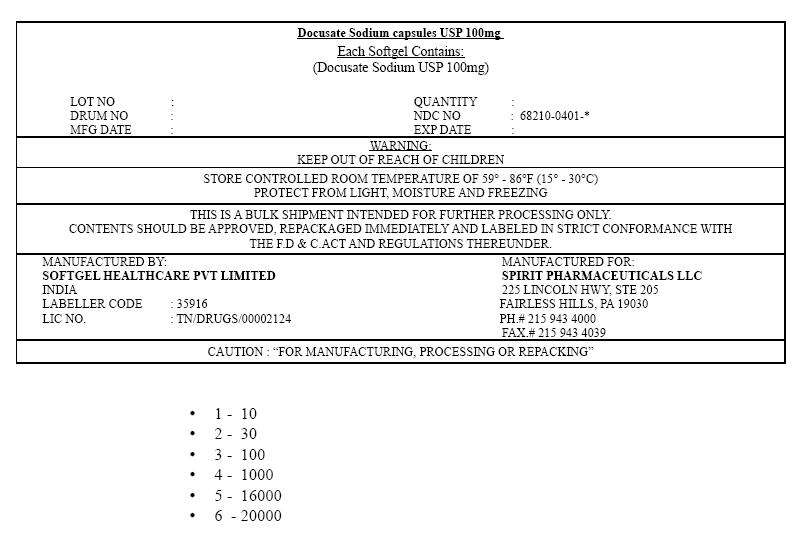
PRINCIPAL DISPLAY PANEL
Docusate Sodium capsules USP 100mg
Each Softgel Contains:
(Docusate Sodium USP 100mg)
| LOT NO | : | QUANTITY | : | ||
| DRUM NO | : | NDC NO | : 68210-0401-* | ||
| MFG DATE | : | EXP DATE | : |
WARNING:
KEEP OUT OF REACH OF CHILDREN
STORE CONTROLLED ROOM TEMPERATURE OF 59° – 86°F (15° – 30°C)
PROTECT FROM LIGHT, MOISTURE AND FREEZING
THIS IS A BULK SHIPMENT INTENDED FOR FURTHER PROCESSING ONLY.
CONTENTS SHOULD BE APPROVED, REPACKAGED IMMEDIATELY AND LABELED IN STRICT CONFORMANCE WITH
THE F.D & C.ACT AND REGULATIONS THEREUNDER.
MANUFACTURED BY:
SOFTGEL HEALTHCARE PVT LIMITED
INDIA
LABELLER CODE : 35916
LIC NO. : TN/DRUGS/00002124
MANUFACTURED FOR:
SPIRIT PHARMACEUTICALS LLC
225 LINCOLN HWY, STE 205
FAIRLESS HILLS, PA 19030
PH.# 215 943 4000
FAX.# 215 943 4039
CAUTION : "FOR MANUFACTURING, PROCESSING OR REPACKING"
- 1 - 10
- 2 - 30
- 3 - 100
- 4 - 1000
- 5 - 16000
- 6 - 20000
DOCUSATE SODIUMDOCUSATE SODIUM CAPSULE, LIQUID FILLED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||