DIFFERIN
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DIFFERIN safely and effectively. See full prescribing information for DIFFERIN (adapalene) Gel, 0.3% DIFFERIN (adapalene) Gel, 0.3% For topical use only Initial U.S. Approval: 1996INDICATIONS AND USAGEDIFFERIN Gel, 0.3%, is a retinoid, indicated for the topical treatment of acne vulgaris in patients 12 years of age and older. (1)DOSAGE AND ADMINISTRATIONApply a thin film of DIFFERIN Gel, 0.3% to the entire face and any other affected areas of the skin once daily in the evening, after washing gently with a non-medicated soap. (2) For topical use only. Not for ophthalmic, oral or intravaginal use. (2)DOSAGE FORMS AND STRENGTHSEach gram of DIFFERIN Gel, 0.3% contains 3 mg adapalene in an off-white aqueous gel. (3)CONTRAINDICATIONSDIFFERIN Gel, 0.3% should not be administered to individuals who are hypersensitive to adapalene or any of the components in the gel vehicle. (4)WARNINGS AND PRECAUTIONSUltraviolet Light and Environmental Exposure: Avoid exposure to sunlight and sunlamps. Wear sunscreen when sun exposure cannot be avoided. (5.1) Erythema, scaling, dryness, and stinging/burning were reported with use of DIFFERIN Gel. (5.2)Side EffectsThe most frequently reported (≥ 1%) adverse reactions were dry skin, skin discomfort, pruritus, desquamation, and sunburn. ( 6.1) To report SUSPECTED ADVERSE REACTIONS, contact Galderma Laboratories, L.P. at 1-866-735-4137 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSAs DIFFERIN Gel has the potential to induce local irritation in some patients, concomitant use of other potentially irritating topical products (medicated or abrasive soaps and cleansers, soaps and cosmetics that have a strong drying effect, and products with high concentrations of alcohol, astringents, spices, or lime) should be approached with caution. Use with caution, especially when using preparations containing sulfur, resorcinol, or salicylic acid in combination with DIFFERIN Gel. (7)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 DIFFERIN INDICATIONS AND USAGE
- 2 DIFFERIN DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 DIFFERIN CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 DIFFERIN ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 DIFFERIN DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/ STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PACKAGE LABEL
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
DIFFERIN Gel, 0.3% is indicated for the topical treatment of acne vulgaris in patients 12 years of age and older.
2 DOSAGE AND ADMINISTRATION
Apply a thin film of DIFFERIN Gel, 0.3% to the entire face and any other affected areas of the skin once daily in the evening, after washing gently with a non-medicated soap. Avoid application to the areas of skin around eyes, lips, and mucous membranes. A mild transitory sensation of warmth or slight stinging may occur shortly after the application of DIFFERIN Gel, 0.3%. Patients should be instructed to minimize sun exposure. Patients may be instructed to use moisturizers for relief of dry skin or irritation.
If therapeutic results are not noticed after 12 weeks of treatment, therapy should be re-evaluated.
For topical use only. Not for ophthalmic, oral or intravaginal use.
3 DOSAGE FORMS AND STRENGTHS
Each gram of DIFFERIN Gel, 0.3% contains 3 mg adapalene in an off-white aqueous gel.
4 CONTRAINDICATIONS
DIFFERIN Gel, 0.3% should not be administered to individuals who are hypersensitive to adapalene or any of the components in the gel vehicle.
5 WARNINGS AND PRECAUTIONS
5.1 Ultraviolet Light and Environmental Exposure
Exposure to sunlight, including sunlamps, should be minimized during use of DIFFERIN Gel, 0.3%. Patients who normally experience high levels of sun exposure, and those with inherent sensitivity to sun, should be warned to exercise caution. Use of sunscreen products and protective clothing over treated areas is recommended when exposure cannot be avoided. Weather extremes, such as wind or cold, also may be irritating to patients under treatment with DIFFERIN Gel, 0.3%.
5.2 Local Cutaneous Reactions
Certain cutaneous signs and symptoms of treatment such as erythema, scaling, dryness, and stinging/burning were reported with use of DIFFERIN Gel, 0.3%. These were most likely to occur during the first four weeks of treatment, were mostly mild to moderate in intensity, and usually lessened with continued use of the medication. Depending upon the severity of these side effects, patients should be instructed to either use a moisturizer, reduce the frequency of application of DIFFERIN Gel, 0.3% or discontinue use.
Avoid contact with the eyes, lips, angles of the nose, and mucous membranes. The product should not be applied to cuts, abrasions, eczematous or sunburned skin. As with other retinoids, use of "waxing" as a depilatory method should be avoided on skin treated with adapalene.
As DIFFERIN Gel has the potential to induce local irritation in some patients, concomitant use of other potentially irritating topical products (medicated or abrasive soaps and cleansers, soaps and cosmetics that have a strong drying effect and products with high concentrations of alcohol, astringents, spices, or lime) should be approached with caution.
5.3 Allergic/ Hypersensitivity Reactions
Reactions characterized by symptoms such as pruritus, face edema, eyelid edema, and lip swelling, requiring medical treatment have been reported during postmarketing use of adapalene. A patient should stop using DIFFERIN Gel, 0.3% and consult a doctor if experiencing allergic or anaphylactoid/anaphylactic reactions (e.g., skin rash, pruritus, hives, chest pain, edema, and shortness of breath) during treatment.
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reactions rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
In the multi-center, controlled clinical trial, signs and symptoms of local cutaneous irritation were monitored in 258 acne patients who used DIFFERIN Gel, 0.3% once daily for 12 weeks. Of the patients who experienced cutaneous irritation (erythema, scaling, dryness, and/or burning/stinging), the majority of cases were mild to moderate in severity, occurred early in treatment and decreased thereafter. The incidence of local cutaneous irritation with DIFFERIN Gel, 0.3% from the controlled clinical study is provided in the following table:
| Incidence of Local Cutaneous Irritation with DIFFERIN Gel, 0.3% from Controlled Clinical Study (N = 253*) Maximum Severity Scores Higher Than Baseline |
|||
|---|---|---|---|
| Mild | Moderate | Severe | |
| Erythema | 66 (26.1%) | 33 (13.0%) | 1 (0.4%) |
| Scaling | 110 (43.5%) | 47 (18.6%) | 3 (1.2%) |
| Dryness | 113 (44.7%) | 43 (17.0%) | 2 (0.8%) |
| Burning/Stinging | 72 (28.5%) | 36 (14.2%) | 9 (3.6%) |
* Total number of subjects with local cutaneous data for at least one post-Baseline evaluation.
| DIFFERIN (adapalene) Gel, 0.3% | Vehicle Gel | |
|---|---|---|
| N=258 | N=134 | |
| Related* Adverse Reactions | 57 (22.1%) | 6 (4.5%) |
| Dry Skin | 36 (14%) | 2 (1.5%) |
| Skin Discomfort | 15 (5.8%) | 0 (0.0%) |
| Desquamation | 4 (1.6%) | 0 (0.0%) |
* Selected adverse reactions defined by investigator as Possibly, Probably or Definitely Related
Related adverse reactions from the controlled clinical trial that occurred in greater than 1% of patients who used DIFFERIN Gel, 0.3% once daily included: dry skin (14.0%), skin discomfort (5.8%), pruritus (1.9%), desquamation (1.6%), and sunburn (1.2%). The following selected adverse reactions occurred in less than 1% of patients: acne flare, contact dermatitis, eyelid edema, conjunctivitis, erythema, pruritus, skin discoloration, rash, and eczema.
In a one-year, open-label safety study of 551 patients with acne who received DIFFERIN Gel, 0.3%, the pattern of adverse reactions was similar to the 12-week controlled study.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of adapalene: skin irritation, application site pain, face edema, eyelid edema, lip swelling, and angioedema. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate the frequency or establish a causal relationship to drug exposure.
7 DRUG INTERACTIONS
Concomitant Topical Medications
As DIFFERIN Gel, 0.3% has the potential to induce local irritation in some patients, concomitant use of other potentially irritating topical products (medicated or abrasive soaps and cleansers, soaps and cosmetics that have a strong drying effect, and products with high concentrations of alcohol, astringents, spices, or lime) should be approached with caution. Particular caution should be exercised in using preparations containing sulfur, resorcinol, or salicylic acid in combination with DIFFERIN Gel, 0.3%. If these preparations have been used, it is advisable not to start therapy with DIFFERIN Gel, 0.3%, until the effects of such preparations have subsided.
No formal drug-drug interaction studies were conducted with DIFFERIN Gel, 0.3%.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic effects. Pregnancy Category C.
Retinoids may cause fetal harm, when administered to pregnant women. Adapalene has been shown to be teratogenic in rats and rabbits when administered orally (see Animal Data below). There are no adequate and well-controlled studies in pregnant women. DIFFERIN Gel, 0.3% should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. The safety and efficacy of DIFFERIN Gel, 0.3% in pregnancy has not been established.
Human Data
In clinical trials involving DIFFERIN Gel, 0.3% in the treatment of acne vulgaris, women of child-bearing potential initiated treatment only after having had a negative pregnancy test and used effective birth control measures during therapy. However, 6 women treated with DIFFERIN Gel, 0.3% became pregnant. One patient elected to terminate the pregnancy, two patients delivered healthy babies by normal delivery, two patients delivered prematurely and the babies remained in intensive care until reaching a healthy state and one patient was lost to follow-up.
Animal Data
- No teratogenic effects were seen in rats at oral doses of 0.15 to 5.0 mg/kg/day adapalene representing up to 6 times the maximum recommended human dose (MRHD) based on mg/m² comparisons. Adapalene has been shown to be teratogenic in rats and rabbits when administered orally at doses ≥ 25 mg/kg representing 32 and 65 times, respectively, the MRHD based on mg/m² comparisons. Findings included cleft palate, microphthalmia, encephalocele and skeletal abnormalities in the rat and umbilical hernia, exophthalmos and kidney and skeletal abnormalities in the rabbit.
- Cutaneous teratology studies in rats and rabbits at doses of 0.6, 2.0, and 6.0 mg/kg/day exhibited no fetotoxicity and only minimal increases in supernumerary ribs in both species and delayed ossification in rabbits. Systemic exposure (AUC0-24h) to adapalene 0.3% gel at topical doses of 6.0 mg/kg/day in rats and rabbits represented 5.7 and 28.7 times, respectively, the exposure in acne patients treated with adapalene 0.3% gel applied to the face, chest and back (2 grams applied to 1000 cm2 of acne involved skin).
8.3 Nursing Mothers
It is not known whether adapalene is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when DIFFERIN Gel, 0.3% is administered to a nursing woman.
8.4 Pediatric Use
Safety and effectiveness have not been established in pediatric patients below the age of 12.
8.5 Geriatric Use
Clinical studies of DIFFERIN Gel, 0.3% did not include subjects 65 years of age and older to determine whether they respond differently than younger subjects. Safety and effectiveness in geriatric patients age 65 and above have not been established.
10 OVERDOSAGE
DIFFERIN Gel, 0.3% is intended for topical use only. If the medication is applied excessively, no more rapid or better results will be obtained and marked redness, scaling, or skin discomfort may occur. Chronic ingestion of the drug may lead to the same side effects as those associated with excessive oral intake of vitamin A.
11 DESCRIPTION
DIFFERIN (adapalene) Gel, 0.3% contains adapalene 0.3% (3 mg/g) in a topical aqueous gel for use in the treatment of acne vulgaris, consisting of carbomer 940, edetate disodium, methylparaben, poloxamer 124, propylene glycol, purified water, and sodium hydroxide. May contain hydrochloric acid for pH adjustment.
The chemical name of adapalene is 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic acid. It is a white to off-white powder, which is soluble in tetrahydrofuran, very slightly soluble in ethanol, and practically insoluble in water. The molecular formula is C28H28O3 and molecular weight is 412.53. Adapalene is represented by the following structural formula.
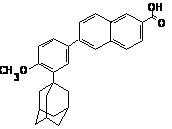
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Adapalene binds to specific retinoic acid nuclear receptors but does not bind to cytosolic receptor protein. Biochemical and pharmacological profile studies have demonstrated that adapalene is a modulator of cellular differentiation, keratinization, and inflammatory processes. However, the significance of these findings with regard to the mechanism of action of adapalene for the treatment of acne is unknown.
12.2 Pharmacodynamics
Clinical pharmacodynamic studies have not been conducted for Differin Gel, 0.3%.
12.3 Pharmacokinetics
Systemic exposure of adapalene following topical application of DIFFERIN Gel was evaluated in a clinical study. Sixteen acne patients were treated once daily for 10 days with 2 grams of DIFFERIN Gel, 0.3% applied to the face, chest and back, corresponding to approximately 2 mg/cm2. Fifteen patients had quantifiable (LOQ = 0.1 ng/mL) adapalene levels resulting in a mean Cmax of 0.553 ± 0.466 ng/mL on Day 10 of treatment. The mean AUC0-24hr was 8.37 ± 8.46 ng.h/mL as determined in 15 of the 16 patients on Day 10. The terminal apparent half-life, determined in 15 of 16 patients, ranged from 7 to 51 hours, with a mean of 17.2 ± 10.2 hours. Adapalene was rapidly cleared from plasma and was not detected 72 hours after the last application for all but one subject. Exposure of potential circulating metabolites of adapalene was not measured. Excretion of adapalene appears to be primarily by the biliary route.
In another clinical study in patients with moderate to moderately severe acne, DIFFERIN (adapalene) Gel, 0.3% or Adapalene Gel, 0.1% was applied to the face and optionally to the trunk, once daily for 12 weeks. Seventy-eight (78) patients had plasma adapalene levels evaluated at Weeks 2, 8, and 12. Of the 209 plasma samples analyzed, adapalene concentrations were below the limit of detection (LOD = 0.15 ng/mL) of the method in all samples but three. For the three samples, traces of adapalene below the limit of quantification (LOQ = 0.25 ng/mL) of the method were found. One of these samples was taken at Week 12 from a male patient treated with DIFFERIN Gel, 0.3% who treated the face and the trunk for eight weeks (thereafter, only the face was treated). The second and third samples were from the Week 2 and 12 visits of a female patient treated with Adapalene Gel, 0.1% who treated only the face for 12 weeks. In this study, the average daily usage of product was 1 g/day.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies with adapalene have been conducted in mice at topical doses of 0.4, 1.3, and 4.0 mg/kg/day, and in rats at oral doses of 0.15, 0.5, and 1.5 mg/kg/day. These doses are up to 3 times (mice) and 2 times (rats) in terms of mg/m²/day the potential exposure at the maximum recommended human dose (MRHD), assumed to be 2.5 grams DIFFERIN Gel, 0.3%. In the oral study, increased incidence of benign and malignant pheochromocytomas in the adrenal medullas of male rats was observed.
No photocarcinogenicity studies were conducted. Animal studies have shown an increased risk of skin neoplasms with the use of pharmacologically similar drugs (e.g., retinoids) when exposed to UV irradiation in the laboratory or to sunlight. Although the significance of these studies to human use is not clear, patients should be advised to avoid or minimize exposure to either sunlight or artificial UV irradiation sources.
Adapalene did not exhibit mutagenic or genotoxic effects in vitro (Ames test, Chinese hamster ovary cell assay, mouse lymphoma TK assay) and in vivo (mouse micronucleus test).
Reproductive function and fertility studies were conducted in rats administered oral doses of adapalene in amounts up to 20 mg/kg/day (up to 26 times the MRHD based on mg/m² comparisons). No effects of adapalene were found on the reproductive performance or fertility of the F0 males or females. There were also no detectable effects on the growth, development and subsequent reproductive function of the F1 offspring.
14 CLINICAL STUDIES
The safety and efficacy of once daily use of DIFFERIN Gel, 0.3% for treatment of acne vulgaris were assessed in one 12 week, multi-center, controlled, clinical study, conducted in a total of 653 patients 12 to 52 years of age with acne vulgaris of mild to moderate severity. All female patients of child-bearing potential enrolled in the study were required to have a negative urine pregnancy test at the beginning of the study and were required to practice a highly effective method of contraception during the study. Female patients who were pregnant, nursing or planning to become pregnant were excluded from the study.
Patients enrolled in the study were Caucasian (72%), Hispanic (12%), African-American (10%), Asian (3%), and other (2%). An equal number of males (49.5%) and females (50.5%) enrolled. Success was defined as "Clear" or "Almost Clear" in the Investigator’s Global Assessment (IGA). The success rate, mean reduction, and percent reduction in acne lesion counts from Baseline after 12 weeks of treatment are presented in the following table:
| DIFFERIN (adapalene) Gel, 0.3% | Adapalene Gel, 0.1% | Vehicle Gel | ||
|---|---|---|---|---|
| N=258 | N=261 | N=134 | ||
| IGA Success Rate | 53 (21%) | 41 (16%) | 12 (9%) | |
| Inflammatory Lesions | ||||
| Mean Baseline Count | 27.7 | 28.1 | 27.2 | |
| Mean Absolute (%) Reduction | 14.4 (51.6%) | 13.9 (49.7%) | 11.2 (40.7%) | |
| Non-inflammatory Lesions | ||||
| Mean Baseline Count | 39.4 | 41.0 | 40.0 | |
| Mean Absolute (%) Reduction | 16.3 (39.7%) | 15.2 (35.2%) | 10.3 (27.2%) | |
| Total Lesions | ||||
| Mean Baseline Count | 67.1 | 69.1 | 67.2 | |
| Mean Absolute (%) Reduction | 30.6 (45.3%) | 29.0 (41.8%) | 21.4 (33.7%) | |
16 HOW SUPPLIED/ STORAGE AND HANDLING
DIFFERIN Gel, 0.3% is supplied in the following size.
45g tube – NDC 54868-5958-0
Storage : Store at controlled room temperature 68° to 77°F (20° to 25°C) with excursions permitted between 59° to 86°F (15° to 30°C). Protect from freezing. Keep out of reach of children.
17 PATIENT COUNSELING INFORMATION
17.1 Information for Patients
Patients using DIFFERIN Gel, 0.3%, should receive the following information and instructions:
- This medication is to be used only as directed by the physician.
- Do not use more than the recommended amount and do not apply more than once daily as this will not produce faster results, but may increase irritation.
- Apply a thin film of DIFFERIN Gel, 0.3% to the entire face and any other affected areas of the skin once daily in the evening, after washing gently with a non-medicated soap.
- Avoid contact with the eyes, lips, angles of the nose, and mucous membranes.
- Moisturizers may be used if necessary; however, products containing alpha hydroxy or glycolic acids should be avoided.
- Exposure of the eye to this medication may result in reactions such as swelling, conjunctivitis, and eye irritation.
- This medication should not be applied to cuts, abrasions, eczematous, or sunburned skin.
- Wax depilation should not be performed on treated skin due to the potential for skin erosions.
- Minimize exposure to sunlight including sunlamps. Recommend the use of sunscreen products and protective apparel (e.g., hat) when exposure cannot be avoided.
- During the early weeks of therapy, an apparent exacerbation of acne may occur. This may be due to the action of the medication on previously unseen lesions and should not be considered a reason to discontinue therapy.
- Contact the doctor if skin rash, pruritus, hives, chest pain, edema, and shortness of breath occurs, as these may be signs of allergy or hypersensitivity.
- It is for external use only.
Marketed by:
GALDERMA LABORATORIES, L.P.
Fort Worth, Texas 76177 USA
Manufactured by:
DPT Laboratories, Ltd.
San Antonio, Texas 78215 USA
GALDERMA is a registered trademark.
325089-0810
Manufactured by:
G Production Inc.
Baie d’Urfe´, QC, H9X 3S4 Canada
Made in Canada
GALDERMA is a registered trademark.
P51238-1
Additional barcode labeling by:
Physicians Total Care, Inc.
Tulsa, Oklahoma 74146
PACKAGE LABEL

Differin® 0.3
(adapalene)gel, 0.3%
GEL
0.3%
NDC 54868-5958-0
Rx Only
NET WT. 45 g
For external use only. Not for ophthalmic, oral, or intravaginal use.
Usual dosage: apply a thin film once a day at nighttime to affected areas. See package insert for complete prescribing information.
Each gram contains: adapalene 0.3% (3 mg) in an aqueous gel consisting of carbomer 940, edetate disodium, methylparaben, poloxamer 124, propylene glycol, purified water, and sodium hydroxide. May contain hydrochloric acid for pH adjustment.
Storage: Store at controlled room temperature 68° to 77°F (20° to 25°C) with excursions permitted between 59° to 86°F (15° to 30°C). Protect from freezing.
DIFFERINadapalene GEL
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||