Despec
International Ethical Labs
Great Southern Laboratories
Despec EDA Drops
FULL PRESCRIBING INFORMATION: CONTENTS*
- Drug Facts
- Despec Uses
- Warnings
- Directions
- Despec Other information
- Inactive ingredients
- Questions? Comments?
- Product Packaging
FULL PRESCRIBING INFORMATION
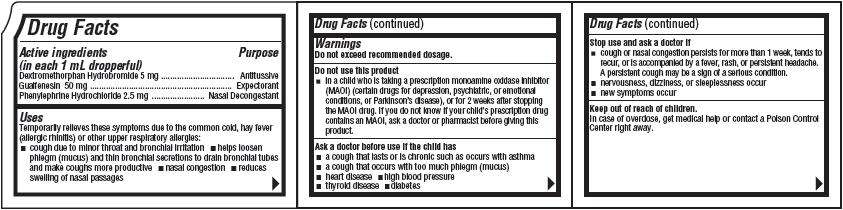
Drug Facts
Active ingredients(in each 1 mL dropperful)
Purpose
Despec Uses
- cough due to minor throat and bronchial irritation
- helps loosen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes and make coughs more productive
- nasal congestion
- reduces swelling of nasal passages
Warnings
Do not exceed recommended dosage.Do not use this product
- in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child's prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
Ask a doctor before use if the child has
- a cough that lasts or is chronic such as occurs with asthma
- a cough that occurs with too much phlegm (mucus)
- heart disease
- high blood pressure
- thyroid disease
- diabetes
Stop use and ask a doctor if
- cough or nasal congestion persists for more than 1 week, tends to recur, or is accompanied by a fever, rash, or persistent headache. A persistent cough may be a sign of a serious condition.
- nervousness, dizziness, or sleeplessness occur
- new symptoms occur
Keep out of reach of children.
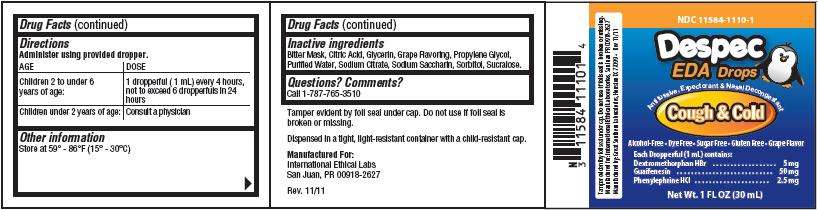
Directions
Administer using provided dropper.| AGE |
DOSE |
| Children 2 to under 6 years of age: |
1 dropperful (1 mL) every 4 hours, not to exceed 6 dropperfuls in 24 hours |
| Children under 2 years of age: |
Consult a physician |
Despec Other information
Inactive ingredients
Questions? Comments?
Manufactured For:
Product Packaging
Despec
EDA Drops
Cough & Cold
Do not use if foil seal is broken or missing.
International Ethical Laboratories,

DespecDextromethorphan Hydrobromide, Guaifenesin, Phenylephrine Hydrochloride LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!