Corvita
CORVITA™ Multivitamin & Mineral Supplement with Iron
FULL PRESCRIBING INFORMATION: CONTENTS*
- CORVITA DESCRIPTION
- CORVITA INDICATIONS AND USAGE
- CORVITA CONTRAINDICATIONS
- PRECAUTIONS
- DRUG INTERACTIONS
- CORVITA ADVERSE REACTIONS
- CORVITA DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- STORAGE
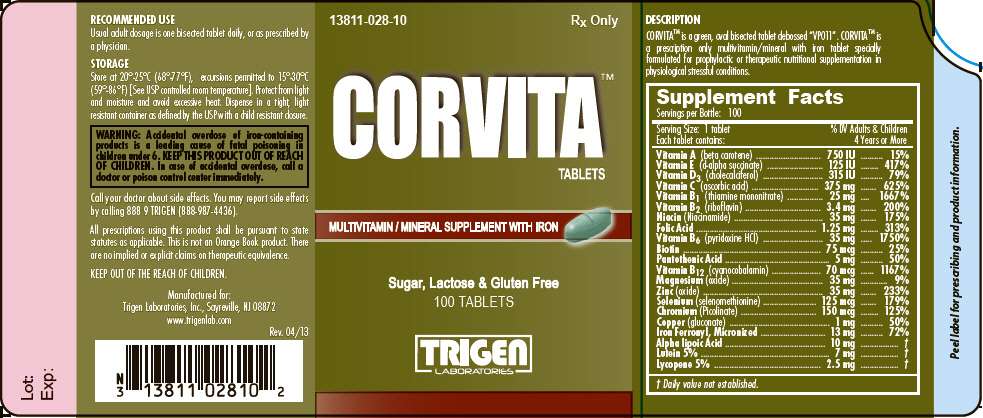
- PRINCIPAL DISPLAY PANEL - 100 Tablet Bottle Label
FULL PRESCRIBING INFORMATION
Sugar, Lactose & Gluten Free
Rx Only
CORVITA DESCRIPTION
CORVITA™ is a green, oval bisected tablet debossed "VP011". CORVITA™ is a prescription only multivitamin/mineral with iron tablet specially formulated for prophylactic or therapeutic nutritional supplementation in physiological stressful conditions.
| Supplement Facts Servings per Bottle: 100 |
||
|---|---|---|
| Serving Size: 1 tablet Each tablet contains: |
% DV Adults & Children 4 Years or More |
|
| Vitamin A (beta carotene) | 750 IU | 15% |
| Vitamin E (d-alpha succinate) | 125 IU | 417% |
| Vitamin D3 (cholecalciferol) | 315 IU | 79% |
| Vitamin C (ascorbic acid) | 375 mg | 625% |
| Vitamin B1 (thiamine mononitrate) | 25 mg | 1667% |
| Vitamin B2 (riboflavin) | 3.4 mg | 200% |
| Niacin (Niacinamide) | 35 mg | 175% |
| Folic Acid | 1.25 mg | 313% |
| Vitamin B6 (pyridoxine HCl) | 35 mg | 1750% |
| Biotin | 75 mcg | 25% |
| Pantothenic Acid | 5 mg | 50% |
| Vitamin B12 (cyanocobalamin) | 70 mcg | 1167% |
| Magnesium (oxide) | 35 mg | 9% |
| Zinc (oxide) | 35 mg | 233% |
| Selenium (selenomethionine) | 125 mcg | 179% |
| Chromium (Picolinate) | 150 mcg | 125% |
| Copper (gluconate) | 1 mg | 50% |
| Iron Ferronyl, Micronized | 13 mg | 72% |
| Alpha lipoic Acid | 10 mg |
|
| Lutein 5% | 7 mg |
 |
| Lycopene 5% | 2.5 mg |
 |
Inactive Ingredients: silicified microcrystalline cellulose, microcrystalline cellulose, croscarmellose sodium, hypromellose, titanium dioxide, fumed silica, tripotassium citrate, stearic acid, magnesium stearate, citric acid, polyethylene glycol, ethyl cellulose, talc, polysorbate 80, FD&C yellow #5 lake, vegetable oil, black iron oxide, hydroxypropyl cellulose, FD&C blue #1 lake.
CORVITA INDICATIONS AND USAGE
CORVITA™ Multivitamins/Minerals with Iron tablets are indicated for the distinctive nutritional requirements of persons being treated for vitamin deficiencies by a physician.
CORVITA CONTRAINDICATIONS
CORVITA™ Multivitamin/Mineral with Iron tablets should not be used by patients with known history of hypersensitivity to any of the listed ingredients or as directed by your physician.
| WARNING |
| Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately. |
PRECAUTIONS
Folic acid when taken in doses over 0.1 mg daily, may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations remain progressive.
DRUG INTERACTIONS
CORVITA™ Multivitamins/Mineral with Iron tablets are not recommended for and should not be given to patients receiving levodopa because the action of levodopa is antagonized by pyridoxine.
CORVITA ADVERSE REACTIONS
Adverse reactions have been reported with specific vitamins and minerals but generally at levels substantially higher than those contained in CORVITA™. However, allergic and idiosyncratic reactions are possible at lower levels. Iron, even at the usual recommended levels, has been associated with gastrointestinal intolerance in some patients.
CORVITA DOSAGE AND ADMINISTRATION
Usual adult dosage is one bisected tablet daily, or as prescribed by a physician
HOW SUPPLIED
CORVITA™ tablets are supplied in bottles of 100 tablets.
Product Code: 13811-028-10
STORAGE
Store at 20°-25°C (68°-77°F), excursions permitted to 15°-30°C (59°-86°F) [See USP controlled room temperature]. Protect from light and moisture and avoid excessive heat. Dispense in a tight, light resistant container as defined by the USP with a child resistant closure..
Call your doctor about side effects. You may report side effects by calling 888 9 TRIGEN (888-987-4436).
All prescriptions using this product shall be pursuant to statutes as applicable. This is not an Orange Book product. There are no implied or explicit claims on therapeutic equivalence.
KEEP OUT OF REACH OF CHILDREN.
Rx Only
Manufactured for:
TRIGEN Laboratories, Inc., Sayreville, NJ 08872
www.trigenlab.com
Rev. 04/13
PRINCIPAL DISPLAY PANEL - 100 Tablet Bottle Label
13811-028-10
Rx Only
CORVITA™
TABLETS
MULTIVITAMIN / MINERAL SUPPLEMENT WITH IRON
Sugar, Lactose & Gluten Free
100 TABLETS
TRIGEN
LABORATORIES
Corvita.beta.-carotene, .ALPHA.-TOCOPHEROL SUCCINATE, D-, cholecalciferol, ascorbic acid, thiamine mononitrate, riboflavin, niacinamide, folic acid, pyridoxine Hydrochloride, Biotin, Pantothenic Acid, cyanocobalamin, Magnesium oxide, Zinc oxide, Selenomethionine, Chromium picolinate, Copper gluconate, .Alpha.-Lipoic Acid, Lutein, and Lycopene TABLET, COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||