Cormax
FULL PRESCRIBING INFORMATION: CONTENTS*
- Cormax® Ointment 0.05% (Clobetasol Propionate Ointment, USP)
- CORMAX DESCRIPTION
- CLINICAL PHARMACOLOGY
- Pharmacokinetics
- CORMAX INDICATIONS AND USAGE
- CORMAX CONTRAINDICATIONS
- PRECAUTIONS
- Information for Patients
- Laboratory Tests
- Carcinogenesis, Mutagenesis, Impairment of Fertility
- Pregnancy
- Nursing Mothers
- Pediatric Use
- Geriatric Use
- CORMAX ADVERSE REACTIONS
- OVERDOSAGE
- CORMAX DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Cormax® Ointment 0.05% (Clobetasol Propionate Ointment, USP)
Rx onlyFor Dermatologic Use Only - Not for Ophthalmic Use.
CORMAX DESCRIPTION
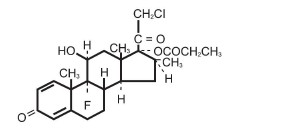 25325
25325CLINICAL PHARMACOLOGY
Pharmacokinetics
DOSAGE AND ADMINISTRATIONDOSAGE AND ADMINISTRATION
CORMAX INDICATIONS AND USAGE
CORMAX CONTRAINDICATIONS
PRECAUTIONS
General: Clobetasol propionate is a highly potent topical corticosteroid that has been shown to suppress the HPA axis at doses as low as 2 g per day.PRECAUTIONS: Pediatric Use
Information for Patients
- This medication is to be used as directed by the physician and should not be used longer than the prescribed time period. It is for external use only Avoid contact with the eyes.
- This medication should not be used for any disorder other than that for which it is prescribed.
- The treated skin area should not be bandaged or otherwise covered or wrapped so as to be occlusive.
- Patients should report any signs of local adverse reactions to the physician.
Laboratory Tests
Carcinogenesis, Mutagenesis, Impairment of Fertility
Pregnancy
Teratogenic Effects: Pregnancy Category C:Nursing Mothers
not
Pediatric Use
Pediatric patients may demonstrate greater susceptibility to topical corticosteroid-induced HPA axis suppression and Cushing's syndrome than mature patients because of a larger skin surface area to body weight ratio.
Geriatric Use
CORMAX ADVERSE REACTIONS
OVERDOSAGE
PRECAUTIONSCORMAX DOSAGE AND ADMINISTRATION
treatment must be limited to two consecutive weeks, and amounts greater than 50 g per week should not be used, Cormax Ointment is not to be used with occlusive dressings.
HOW SUPPLIED
Rx only
Keep out of reach of children
Principal Display Panel
New NDC#NDC 52544-048-89 45g Tube
Cormax Ointment 0.05%
(Clobetasol Propionate Ointment, USP)
Watson Rx Only

Cormaxclobetasol propionate OINTMENT
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!