Citrus Burst Antibacterial Cleansing Hand Wash
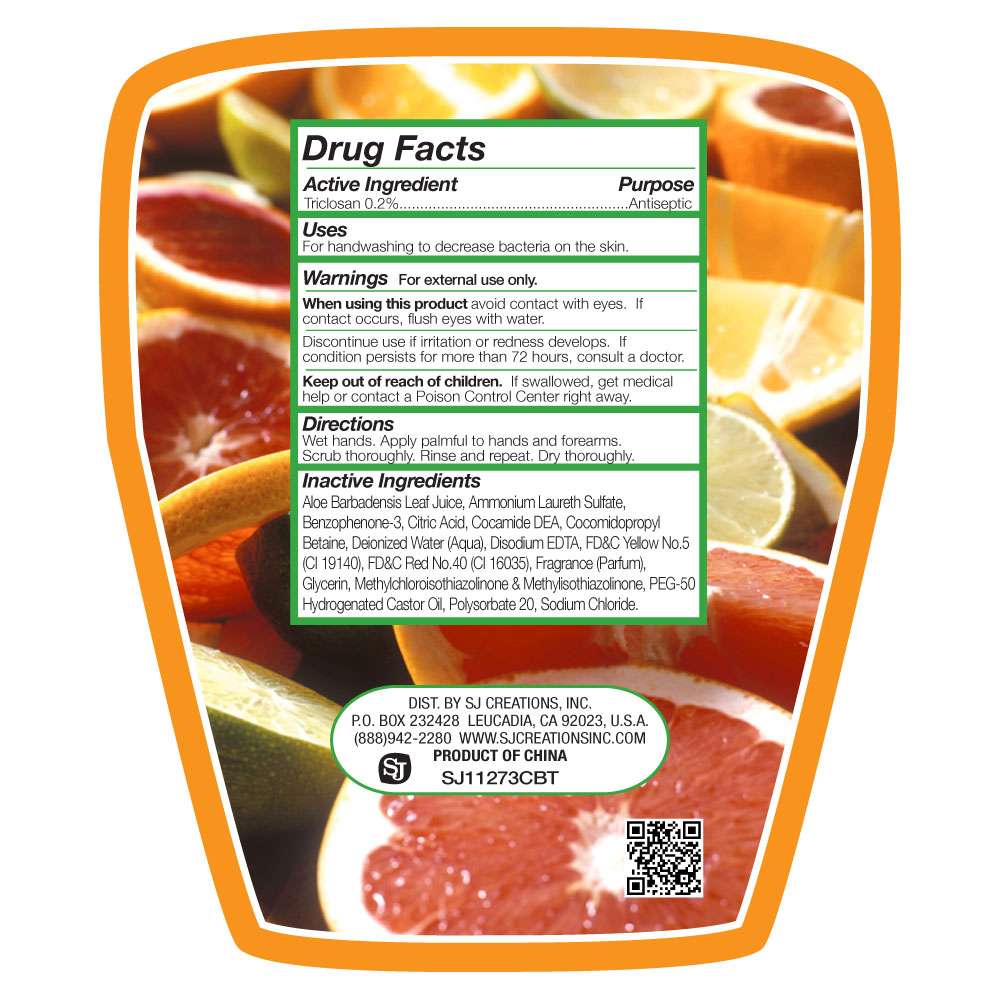
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Citrus Burst Antibacterial Cleansing Hand Wash Uses
- Warnings
- When using this product
- Keep out of the reach of Children
- Directions
- Inactive Ingredients
FULL PRESCRIBING INFORMATION
Active Ingredient
Triclosan 0.2% (Purpose: Antiseptic)
Citrus Burst Antibacterial Cleansing Hand Wash Uses
For handwashing to decrease bacteria on the skin.
Warnings
For external use only.
When using this product
Avoid contact with eyes. If contact occurs flush eyes with water.
Discontinue use if irritation or redness develops. If condition persists for more than 72 hours, consult a doctor.
Keep out of the reach of Children
If swallowed get medical help or contact a Poison Control Center right away.
Directions
Wet hands. Apply palmful to hands and forearms. Scrub thoroughly. Rinse and repeat. Dry thoroughly.
Inactive Ingredients
Aloe Barbadensis, Ammonium Laureth Sulfate, Benzophenone-3, Citric Acid, Cocamide DEA, Cocamidopropyl Betaine, Deionized Water (aqua), Disodium EDTA, FD and C Red No.40(CI16035), FD and C Yellow No.5 (CI19140), Fragrance (Perfume) Glycerin, Methylchloroisothiazolinone and
Methylisothiazolinone, PEG-50 Hydrogenated Castor Oil, Polysorbate 20, Sodium Chloride.

DIST. BY SJ CREATIONS, INC.
P.O. BOX 232428 LEUCADIA, CA 92023, U.S.A.
(888)942-2280 WWW.SJCREATIONSINC.COM
PRODUCT OF CHINA
Citrus Burst Antibacterial Cleansing Hand WashTriclosan LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||