Cephalexin
FULL PRESCRIBING INFORMATION: CONTENTS*
- Rx only
- CEPHALEXIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- CEPHALEXIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- CEPHALEXIN ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- PRINCIPAL DISPLAY PANEL 250 mg CAPSULES BOTTLE LABEL
- PRINCIPAL DISPLAY PANEL 500 mg CAPSULES BOTTLE LABEL
FULL PRESCRIBING INFORMATION
Rx only
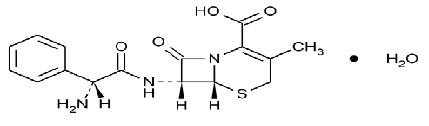
CEPHALEXIN DESCRIPTION
1617342
D
CLINICAL PHARMACOLOGY
Human Pharmacology
Microbiology
In vitroin vitro INDICATIONS AND USAGE
Aerobes, Gram-positive:
Staphylococcus aureus (including penicillinase-producing strains)
Streptococcus pneumoniae (penicillin-susceptible strains)
Streptococcus pyogenes
Aerobes, Gram-negative:
Escherichia coli
Haemophilus influenzae
Klebsiella pneumoniae
Moraxella (Branhamella) catarrhalis
Proteus mirabilis
NoteEnterococcus faecalisStreptococcus faecalisEnterobacterMorganella morganii,Proteus vulgarisPseudomonasAcinetobacter calcoaceticusStreptococcus pneumoniae
Susceptibility Tests
Dilution techniques 1 to 3
|
MIC (mcg/mL)
|
Interpretation
|
|
| ≤8 |
Susceptible |
(S) |
| 16 |
Intermediate |
(I) |
| ≥32 |
Resistant |
(R) |
|
Microorganism
|
MIC (mcg/mL)
|
|
|
E. coli
|
ATCC25922 |
4 to 16 |
|
S. aureus
|
ATCC29213 |
0.12 to 0.5 |
Diffusion techniques 2,3
|
Zone Diameter (mm)
|
Interpretation
|
|
| ≥18 |
Susceptible |
(S) |
| 15 to 17 |
Intermediate |
(I) |
| ≤14 |
Resistant |
(R) |
Interpretation should be as stated above for results using dilution techniques. Interpretation involves correlation of the diameter obtained in the disk test with the MIC for cephalexin.
|
Microorganism
|
Zone Diameter (mm)
|
|
|
E. coli
|
ATCC25922 |
15 to 21 |
|
S. aureus
|
ATCC25923 |
29 to 37 |
INDICATIONS & USAGE
Streptococcus pneumoniaeStreptococcus pyogenes
Otitis media due to Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, Streptococcus pyogenes, and Moraxella catarrhalis
Skin and skin structure infections caused by Staphylococcus aureus and/or Streptococcus pyogenes
Bone infections caused by Staphylococcus aureus and/or Proteus mirabilis
Escherichia coliProteus mirabilisKlebsiella pneumoniae
Note
CEPHALEXIN CONTRAINDICATIONS
WARNINGS
BEFORE THERAPY WITH CEPHALEXIN IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEPHALEXIN, CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF THIS PRODUCT IS TO BE GIVEN TO PENICILLIN-SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS-HYPERSENSITIVITY AMONG BETA-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF AN ALLERGIC REACTION TO CEPHALEXIN OCCURS, DISCONTINUE THE DRUG. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS ANTIHISTAMINES, CORTICOSTEROIDS, PRESSOR AMINES AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.
Clostridium difficile associated diarrhea (CDAD) C.difficile
CdifficileCdifficile
C. diffcileC. diffcile
PRECAUTIONS
General
Prescribing cephalexin in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Patients should be followed carefully so that any side effects or unusual manifestations of drug idiosyncrasy may be detected. If an allergic reaction to cephalexin occurs, the drug should be discontinued and the patient treated with the usual agents (e.g., epinephrine or other pressor amines, antihistamines, or corticosteroids).
Prolonged use of cephalexin may result in the overgrowth of nonsusceptible organisms. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate measures should be taken.
Positive direct Coombs’ tests have been reported during treatment with the cephalosporin antibiotics. In hematologic studies or in transfusion cross-matching procedures when antiglobulin tests are performed on the minor side or in Coombs’ testing of newborns whose mothers have received cephalosporin antibiotics before parturition, it should be recognized that a positive Coombs’ test may be due to the drug.
Cephalexin should be administered with caution in the presence of markedly impaired renal function. Under such conditions, careful clinical observation and laboratory studies should be made because safe dosage may be lower than that usually recommended.
Indicated surgical procedures should be performed in conjunction with antibiotic therapy.
Broad-spectrum antibiotics should be prescribed with caution in individuals with a history of gastrointestinal disease, particularly colitis.
Information for Patients
Patients should be counseled that antibacterial drugs including cephalexin should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When cephalexin is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by cephalexin or other antibacterial drugs in the future.
Drug Interactions
Metformin — In healthy subjects given single 500 mg doses of cephalexin and metformin, plasma metformin mean Cmax and AUC increased by an average of 34% and 24%, respectively, and metformin mean renal clearance decreased by 14%. No information is available about the interaction of cephalexin and metformin following multiple doses of either drug.
Although not observed in this study, adverse effects could potentially arise from co-administration of cephalexin and metformin by inhibition of tubular secretion via organic cationic transporter systems. Accordingly, careful patient monitoring and dose adjustment of metformin is recommended in patients concomitantly taking cephalexin and metformin.
Probenecid
Drug & OR Laboratory Test Interactions
®
Carcinogenesis & Mutagenesis & Impairment Of Fertility
2
Pregnancy
Teratogenic effects Pregnancy Category B2
Nursing Mothers
Pediatric Use
DOSAGE AND ADMINISTRATION
Geriatric Use
Of the 701 subjects in 3 published clinical studies of cephalexin, 433 (62%) were 65 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
see PRECAUTIONS , General
CEPHALEXIN ADVERSE REACTIONS
Gastrointestinal — Onset of pseudomembranous colitis may occur during or after antibacterial treatment. (See WARNINGS .) Nausea and vomiting have been reported rarely. The most frequent side effect has been diarrhea. It was very rarely severe enough to warrant cessation of therapy. Dyspepsia, gastritis, and abdominal pain have also occurred. As with some penicillins and some other cephalosporins, transient hepatitis and cholestatic jaundice have been reported rarely.
Hypersensitivity — Allergic reactions in the form of rash, urticaria, angioedema, and, rarely, erythema multiforme, Stevens-Johnson syndrome, or toxic epidermal necrolysis have been observed. These reactions usually subsided upon discontinuation of the drug. In some of these reactions, supportive therapy may be necessary. Anaphylaxis has also been reported.
Other reactions have included genital and anal pruritus, genital moniliasis, vaginitis and vaginal discharge, dizziness, fatigue, headache, agitation, confusion, hallucinations, arthralgia, arthritis, and joint disorder. Reversible interstitial nephritis has been reported rarely. Eosinophilia, neutropenia, thrombocytopenia, hemolytic anemia, and slight elevations in AST and ALT have been reported.
Adverse Reactions — Fever, colitis, aplastic anemia, hemorrhage, renal dysfunction, and toxic nephropathy.
see INDICATIONS AND USAGE and PRECAUTIONS General
Altered Laboratory Tests
OVERDOSAGE
Signs and Symptoms
TreatmentPhysicians’ Desk ReferencePDR
DOSAGE & ADMINISTRATION
Adults
Pediatric Patients
HOW SUPPLIED
Cephalexin Capsules, USP are available in:
REFERENCES
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically — Fourth Edition. Approved Standard NCCLS Document Document M7-A4, Vol. 17, No. 2, NCCLS Document M7- A4, Vol. 17, No. 2, NCCLS, Wayne, PA, January, 1997.
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests — Sixth Edition. Approved Standard NCCLS Document M2-A6, Vol. 17, No. 1, NCCLS, Wayne, PA, January, 1997.
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing — Eighth Informational Supplement. Approved Standard NCCLS Document M100-S8, Vol. 18, No. 1, NCCLS, Wayne, PA, January, 1998.
ALKEM LABORATORIES LTD.
PRINCIPAL DISPLAY PANEL 250 mg CAPSULES BOTTLE LABEL
CEPHALEXIN CAPSULES, USP
250 mg
Rx Only
100 Capsules

PRINCIPAL DISPLAY PANEL 500 mg CAPSULES BOTTLE LABEL
CEPHALEXIN CAPSULES, USP
500 mg
Rx Only
Bottle of 500Capsules

CephalexinCephalexin CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CephalexinCephalexin CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||