Carisoprodol, Aspirin and Codeine Phosphate
Mirror Pharmaceuticals LLC
Mirror Pharmaceuticals LLC
CARISOPRODOL, ASPIRIN AND CODEINE PHOSPHATE Tablets, USP200mg/325mg/16mgRx Only
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING: May be habit forming. CIII
- CARISOPRODOL, ASPIRIN AND CODEINE PHOSPHATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CARISOPRODOL, ASPIRIN AND CODEINE PHOSPHATE INDICATIONS AND USAGE
- CARISOPRODOL, ASPIRIN AND CODEINE PHOSPHATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- CARISOPRODOL, ASPIRIN AND CODEINE PHOSPHATE ADVERSE REACTIONS
- OVERDOSAGE
- CARISOPRODOL, ASPIRIN AND CODEINE PHOSPHATE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
FULL PRESCRIBING INFORMATION
WARNING: May be habit forming. CIII
CARISOPRODOL, ASPIRIN AND CODEINE PHOSPHATE DESCRIPTION
Carisoprodol, Aspirin and Codeine Phosphate Tablets, USP is a fixed-dose combination product containing the following three products:
- 200 mg of carisoprodol, a centrally-acting muscle relaxant
- 325 mg of aspirin, an analgesic with antipyretic and anti-inflammatory properties
- 16 mg of codeine phosphate, a centrally-acting narcotic analgesic.
It is available as a two-layered, white and yellow, Round tablet for oral administration.
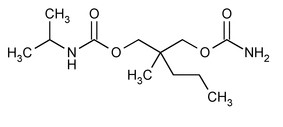
Carisoprodol:Chemically, carisoprodol is N-isopropyl-2-methyl-2-propyl-1,3 propanediol dicarbamate and its molecular formula is C12H24N2O4, with a molecular weight of 260.33. The structural formula of carisoprodol is:
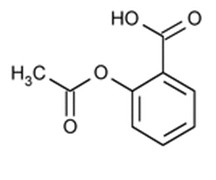
Aspirin:Chemically, aspirin (acetylsalicylic acid) is 2-(acetyloxy)-, benzoic acid and its molecular formula is C9H8O4, with a molecular weight of 180.16. The structural formula of aspirin is:
Codeine Phosphate: Chemically, codeine phosphate is 7,8 Didehydro-4,5α-epoxy-3methoxy-17- ethylmorphinan-6α-ol phosphate (1:1) (salt) hemihydrate and its molecular formula is C18H24NO7P, with a molecular weight of 406.37. The structural formula of codeine phosphate is:

Other ingredients in the Carisoprodol, Aspirin and Codeine Phosphate drug product are FD&C Yellow #5 Aluminum Lake, Corn starch, Hydroxypropyl Cellulose, Lactose Anhydrous, Micro-crystalline Cellulose, Magnesium Stearate, Pregelatinized Starch, Sodium Starch Glycolate and Sodium Lauryl Sulphate.
CLINICAL PHARMACOLOGY
Mechanism of Action
Carisoprodol: The mechanism of action of carisoprodol in relieving discomfort associated with acute painful musculoskeletal conditions has not been clearly identified. In animal studies, muscle relaxation induced by carisoprodol is associated with altered interneuronal activity in the spinal cord and in the descending reticular formation of the brain.
Aspirin: The mechanism of action of aspirin in relieving pain is by inhibition of the body’s production of prostaglandins, which are thought to cause pain sensations by stimulating muscle contractions and dilating blood vessels.
Codeine Phosphate:The precise mechanism of action of codeine phosphate, an opioid agonist, in relieving pain has not been established. The binding of codeine phosphate to mu, delta, and kappa opioid receptors in the central nervous system (CNS) may change the perception of pain. The analgesic activity of codeine phosphate is probably due to its conversion to morphine.
Pharmacodynamics
Carisoprodol: Carisoprodol is a centrally-acting muscle relaxant that does not directly relax tense skeletal muscles. A metabolite of carisoprodol, meprobamate, has anxiolytic and sedative properties. The degree to which these properties of meprobamate contribute to the safety and efficacy of Carisoprodol, Aspirin and Codeine Phosphate is unknown.
Aspirin: Aspirin is a non-¬narcotic analgesic with anti-inflammatory and anti-¬pyretic activity. Inhibition of prostaglandin biosynthesis appears to account for most of its anti¬-inflammatory and for at least part of its analgesic and antipyretic properties. In the CNS, aspirin works on the hypothalamus heat-¬regulating center to reduce fever. Aspirin can cause serious gastrointestinal injury including bleeding, obstruction, and perforations from ulcers possibly by inhibition of the production of prostaglandins, compromising the defenses of the gastric mucosa and the activity of substances involved in tissue repair and ulcer healing (see WARNINGS ). Aspirin inhibits platelet aggregation by irreversibly inhibiting prostaglandin cyclo-oxygenase. This effect lasts for the life of the platelet and prevents the formation of the platelet aggregating factor thromboxane A2.
Codeine Phosphate:Codeine phosphate is a centrally-acting narcotic analgesic. Its actions are qualitatively similar to morphine, but its potency is substantially less. Opioids, including codeine phosphate have the following effects:
- respiratory depression by a direct effect on the brainstem respiratory centers
- depression of the cough reflex by direct effect on the cough center in the medulla
- constriction of the pupils (i.e., miosis)
- decreased gastric, biliary, and pancreatic secretions
- reduction in the motility of the stomach and small and large intestine which results in constipation and delayed digestion
- nausea and vomiting by directly stimulating the chemoreceptor trigger zone
- increased biliary tract pressure as a result of spasm of the sphincter of Oddi
- peripheral vasodilatation which may result in orthostatic hypotension
- histamine release which may result in pruritus, flushing, and sweating
- increased tone of the bladder detrusor muscle, ureters, and vesical sphincter which may result in urinary retention
Pharmacokinetics
Carisoprodol: The pharmacokinetics of carisoprodol and its metabolite meprobamate were studied in a study of 24 healthy subjects (12 male and 12 female) who received single doses of 350 mg of carisoprodol (see Table 1). The Cmax of meprobamate was 2.5 ± 0.5 µg/mL (mean ± SD) after administration of a single 350 mg dose of carisoprodol, which is approximately 30% of the Cmax of meprobamate (approximately 8 µg/mL) after administration of a single 400 mg dose of meprobamate.
| Table 1: Pharmacokinetic Parameters of Carisoprodol and Meprobamate (Mean ± SD, n=24) | ||
|---|---|---|
| Carisoprodol | Meprobamate | |
| Cmax (µg/mL) | 1.8 ± 1.0 | 2.5 ± 0.5 |
| AUCinf(µg•hour/mL) | 7.0 ± 5.0 | 46 ± 9.0 |
| Tmax (hour) | 1.7 ± 0.8 | 4.5 ± 1.9 |
| T1/2 (hour) | 2.0 ± 0.5 | 9.6 ± 1.5 |
Absorption: Absolute bioavailability of carisoprodol has not been determined. After administration of a single dose of 350 mg of carisoprodol, the mean time to peak plasma concentrations (Tmax) of carisoprodol was approximately 1.5 to 2 hours. Co-administration of a high-¬fat meal with 350 mg of carisoprodol had no effect on the pharmacokinetics of carisoprodol.
Metabolism: The major pathway of carisoprodol metabolism is via the liver by cytochrome enzyme CYP2C19 to form meprobamate. This enzyme exhibits genetic polymorphism (see Patients with Reduced CYP2C19 Activity below).
Elimination: Carisoprodol is eliminated by both renal and non¬-renal routes with a terminal elimination half-life of approximately 2 hours after administration of a single dose of 350 mg of carisoprodol. The half-life of meprobamate is approximately 10 hours after administration of a single dose of 350 mg of carisoprodol.
Gender: Exposure of carisoprodol is higher in females than in male subjects (approximately 30 to 50% on a weight adjusted basis). Overall exposure of meprobamate is comparable between female and male subjects.
Patients with Reduced CYP2C19 Activity: Carisoprodol should be used with caution in patients with reduced CYP2C19 activity. Published studies indicate that patients who are poor CYP2C19 metabolizers have a 4-¬fold increase in exposure to carisoprodol, and 50% reduced exposure to meprobamate compared to normal CYP2C19 metabolizers. The prevalence of poor metabolizers in Caucasians and African Americans is approximately 3 to 5% and in Asians is approximately 15 to 20%.
Aspirin:
Absorption: The rate of aspirin absorption from the gastrointestinal (GI) tract is dependent upon the presence or absence of food, gastric pH (the presence or absence of GI antacids), and other physiologic factors. Following absorption, aspirin is hydrolyzed to salicylic acid in the gut wall and during first-¬pass metabolism with peak plasma levels of salicylic acid occurring within 1 to 2 hours of dosing.
Distribution: Salicylic acid is widely distributed to all tissues and fluids in the body including the central nervous system (CNS), breast milk, and fetal tissues. The highest concentrations are found in the plasma, liver, kidneys, heart, and lungs. The protein binding of salicylate is concentration dependent, i.e., nonlinear. At plasma concentrations of salicylic acid < 100 µg/mL and > 400 µg/mL, approximately 90 and 76 percent of plasma salicylate is bound to albumin, respectively.
Metabolism: Aspirin, which has a half-¬life of about 15 minutes, is hydrolyzed in the plasma to salicylic acid such that plasma levels of aspirin may not be detectable 1 to 2 hours after dosing. Salicylic acid, which has a plasma half¬ life of approximately 6 hours, is conjugated in the liver to form salicyluric acid, salicyl phenolic glucuronide, salicyl acyl glucuronide, gentisic acid, and gentisuric acid. At higher serum concentrations of salicylic acid, the total clearance of salicylic acid decreases due to the limited ability of the liver to form both salicyluric acid and phenolic glucuronide. Following toxic doses of aspirin (e.g., > 10 grams), the plasma half¬-life of salicylic acid may be increased to over 20 hours.
Elimination: The elimination of salicylic acid is constant in relation to the plasma salicylic acid concentration. Following therapeutic doses of aspirin, approximately 75, 10, 10, and 5 percent is found excreted in the urine as salicyluric acid, salicylic acid, a phenolic glucuronide of salicylic acid, and an acyl glucuronide of salicylic acid, respectively. As the urinary pH rises above 6.5, the renal clearance of free salicylate increases from less than 5 percent to greater than 80 percent. Alkalinization of the urine is a key concept in the management of salicylate overdose (see OVERDOSAGE, Treatment of Overdosage ). Clearance of salicylic acid is also reduced in patients with renal impairment.
Codeine Phosphate:
Absorption:Codeine is readily absorbed from the GI tract. At therapeutic doses, the analgesic effect reaches a peak within 2 hours and persists between 4 and 6 hours.
Distribution:Codeine is rapidly distributed from the intravascular spaces to the tissues with preferential uptake by the liver, spleen, and kidney. Codeine crosses the blood-brain barrier, and is found in fetal tissue and breast milk. The plasma concentration of codeine does not correlate with brain concentration of codeine or the relief of pain.
Metabolism:The plasma half-life of codeine is about 2.9 hours.
Elimination:The elimination of codeine is primarily via the kidneys, and about 90% of an oral dose is excreted by the kidneys within 24 hours of dosing. The urinary secretion products consist of free and glucuronide-conjugated codeine (about 70%), free and conjugated norcodeine (about 10%), free and conjugated morphine (about 10%), normorphine (4%), and hydrocodone (1%). The remainder of the dose is excreted in the feces.
CARISOPRODOL, ASPIRIN AND CODEINE PHOSPHATE INDICATIONS AND USAGE
Carisoprodol, Aspirin and Codeine Phosphate is indicated for the relief of discomfort associated with acute, painful musculoskeletal conditions in adults. Carisoprodol, Aspirin and Codeine Phosphate should only be used for short periods (up to two or three weeks) because adequate evidence of effectiveness for more prolonged use has not been established and because acute, painful musculoskeletal conditions are generally of short duration (see DOSAGE AND ADMINISTRATION ).
CARISOPRODOL, ASPIRIN AND CODEINE PHOSPHATE CONTRAINDICATIONS
Carisoprodol and Aspirin is contraindicated in patients with a history of:
- a serious GI complication (i.e., bleeding, perforations, obstruction) due to aspirin use
- aspirin induced asthma (a symptom complex which occurs in patients who have asthma, rhinosinusitis, and nasal polyps who develop a severe, potentially fatal bronchospasm shortly after taking aspirin or other NSAIDs)
- hypersensitivity reaction to carbamate such as meprobamate
- acute intermittent prophyria
WARNINGS
Carisoprodol:
Sedation
Carisoprodol has sedative properties and may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a motor vehicle or operating machinery. There have been post-¬marketing reports of motor vehicle accidents associated with the use of carisoprodol.
Since the sedative effects of carisoprodol and other CNS depressants (e.g., alcohol, benzodiazepines, opioids, tricylic antidepressants) may be additive, appropriate caution should be exercised with patients who take more than one of these CNS depressants simultaneously.
Drug Dependence, Withdrawal, and Abuse
In post-¬marketing experience with carisoprodol, cases of dependence, withdrawal, and abuse have been reported with prolonged use. Most cases of dependence, withdrawal, and abuse occurred in patients who have had a history of addiction or who used carisoprodol in combination with other drugs with abuse potential. However, there have been post-¬marketing adverse event reports of carisoprodol¬-associated abuse when used without other drugs with abuse potential. Withdrawal symptoms have been reported following abrupt cessation after prolonged use. To reduce the chance of carisoprodol dependence, withdrawal, or abuse, carisoprodol should be used with caution in addiction prone patients and in patients taking other CNS depressants including alcohol, and carisoprodol should not be used more than two to three weeks for the relief of acute musculoskeletal discomfort.
Carisoprodol, and one of its metabolites, meprobamate (a controlled substance), may cause dependence (see CLINICAL PHARMACOLOGY ).
Aspirin:
Serious Gastrointestinal Adverse Reactions
Aspirin can cause serious gastrointestinal (GI) adverse reactions including bleeding, perforation, and obstruction of the stomach, small intestine, or large intestine, which can be fatal. Aspirin¬-associated serious GI adverse reactions can occur anywhere along the GI tract, at any time, with or without warning symptoms. Patients at higher risk of aspirin-¬associated serious upper GI adverse reactions include patients with a history of aspirin- associated GI bleeding from ulcers (complicated ulcers), a history of aspirin-¬associated ulcers (uncomplicated ulcers), geriatric patients, patients with poor baseline health status, patients taking higher doses of aspirin, and patients taking concomitant anticoagulants, NSAIDs, and/or large amounts of alcohol. To minimize the risk for aspirin-¬associated GI serious adverse reaction, the lowest effective aspirin dose should be used for the shortest possible duration.
Anaphylaxis and Anaphylactoid Reactions
Aspirin may cause an increased risk of serious anaphylaxis and anaphylactoid reactions, which can occur in patients without known prior exposure to aspirin (see
CONTRAINDICATIONS
). Patients with a serious anaphylaxis or anaphylactoid reaction should receive emergency care.
Codeine Phosphate:
Respiratory Depression
Respiratory depression is a serious adverse reaction of opioid agonists, including codeine phosphate. Opioid-associated respiratory depression is more likely to occur in geriatric patients, debilitated patients, in non-tolerant patients who are given large initial doses of opioids, and in patients who are receiving concomitant respiratory depressants (e.g. other opioids, benzodiaze-pines, tricyclic antidepressants, phenothiazines, skeletal muscle relaxants, alcohol). In addition, patients with chronic obstructive pulmonary disease (COPD), restrictive lung disease, decreased respiratory drive, and/or respiratory depression are at a greater risk of opioid-associated respiratory depression. Opioid-associated respiratory depression may be increased in patients with increased intracranial pressure (e.g., patients with head trauma, intracranial lesions).
Abuse and Diversion
Codeine phosphate is a Schedule III controlled substance. Administration of opioids including codeine phosphate has been associated with abuse. Healthcare professionals should contact their State Professional Licensing Board or State Substances Authority for information on how to prevent or detect abuse or diversion of codeine phosphate.
Dependence and Tolerance
Use of opioids, including codeine phosphate, can result in psychological and/or physical depen-dence. Withdrawal symptoms associated with abrupt opioid discontinuation include restlessness, irritability, anxiety, lacrimation, rhinorrhea, sweating, chills, mydriasis, insomnia, diarrhea, tachypnea, tachycardia, and/or hypertension. The use of opioids, including codeine phosphate, use can result in tolerance – the need for increasing doses to maintain a desired effect in the absence of other factors (e.g., disease progression).
Gastrointestinal Obstruction
Opioids, including codeine phosphate, may cause gastrointestinal obstruction.
Sedation
Opioids, including codeine phosphate, may impair the mental and physical abilities required for the performance of potentially hazardous tasks such as driving a motor vehicle or operating ma-chinery. Since the sedative effects of codeine phosphate and other CNS depressants (e.g., other opioids, benzodiazepines, tricyclic antidepressants, skeletal muscle relaxants, alcohol) may be additive, appropriate caution should be exercised with patients who take more than one of these CNS depressants simultaneously.
Hypotension
The use of opioids, including codeine phosphate, may cause hypotension. Opioid-associated hypotension is more likely in patients with dehydration or with the concomitant use of drugs as-sociated with hypotension.
PRECAUTIONS
Patients with impaired renal or hepatic function
The safety and pharmacokinetics of Carisoprodol, Aspirin and Codeine Phosphate in patients with renal or hepatic impairment have not been evaluated.
Carisoprodol:
Since carisoprodol is excreted by the kidney and is metabolized in the liver, caution should be exercised if carisoprodol is administered to patients with impaired renal or hepatic function. Carisoprodol is dialyzable by hemodialysis and peritoneal dialysis.
Seizures
There have been post¬-marketing reports of seizures in patients who received carisoprodol. Most of these cases have occurred in the setting of multiple drug overdoses (including drugs of abuse, illegal drugs, and alcohol) (see
OVERDOSAGE
).
Aspirin:
Gastrointestinal Adverse Reactions
In addition to serious gastrointestinal adverse reactions, the use of aspirin is also associated with gastritis, gastrointestinal erosions, abdominal pain, heartburn, vomiting, and nausea (see
WARNINGS, Serious Gastrointestinal Adverse Reactions
).
Codeine Phosphate:
Obscuring Medical Conditions
Opioids, including codeine phosphate, may obscure the clinical course of patients with head in-juries because of the CNS depressive effects of opioids. In addition, opioids, including codeine phosphate, may obscure the symptoms and/or signs that are used for the diagnosis or for the monitoring of patients with acute abdominal conditions.
Ultra-rapid Metabolizers of Codeine
Some patients may be ultra-rapid metabolizers of codeine phosphate due to a specific CYP2D6*2x2 genotype. These patients convert codeine into its active metabolite, morphine, more rapidly and completely than patients who are normal metabolizers of codeine, resulting in higher than expected serum morphine levels. Even at labeled dosage regimens of codeine phosphate, patients who are ultra-rapid metabolizers may experience overdose symptoms such as respiratory depression, extreme sleepiness, or delirium. Toxic serum levels of morphine have been reported in infants of nursing mothers who may be ultra-rapid metabolizers (see
PRECAUTIONS, Nursing Mothers
). The prevalence of this CYP2D6 phenotype has been esti-mated at 16 to 28%in North Africans, Ethiopians, and Arabs; 1 to 10% in Caucasians; 3% in African Americans; and 0.5 to 1% in Chinese, Japanese, and Hispanics. Data is not available for other ethnic groups. When healthcare providers prescribe codeine-containing products, they should choose the lowest effective dose for the shortest period of time.
Use in Patients with Pancreatic or Biliary Duct Disease
Opioids, including codeine phosphate, should be used with caution in patients with pancreatic or biliary duct disease because opioids may cause spasm of the sphincter of Oddi and diminish pancreatic and/or biliary secretions.
Information for Patients:
Patients should be advised to contact their health care provider if they experience any adverse reactions to Carisoprodol, Aspirin and Codeine Phosphate Tablets.
Carisoprodol:
- Patients should be advised that carisoprodol may cause drowsiness and/or dizziness, and has been associated with motor vehicle accidents. Patients should be advised to avoid taking carisoprodol before engaging in potentially hazardous activities such as driving a motor vehicle or operating machinery (see WARNINGS, Sedation ).
- Patients should be advised to avoid alcoholic beverages while taking carisoprodol and to check with their doctor before taking other CNS depressants such as benzodiazepines, opioids, tricyclic antidepressants, sedating antihistamines, or other sedatives (see WARNINGS, Sedation ).
- Patients should be advised that treatment with carisoprodol should be limited to acute use (up to two or three weeks) for the relief of acute, musculoskeletal discomfort. In the post-marketing experience with carisoprodol, cases of dependence, withdrawal, and abuse have been reported with prolonged use. If the musculoskeletal symptoms still persist, patients should contact their healthcare provider for further evaluation.
Aspirin:
- Patients should be warned that aspirin can cause epigastric discomfort, gastric and duodenal ulcers, and serious GI adverse reactions, such as bleeding, perforation, and/or obstruction of the stomach or intestines, which may result in hospitalization and death. Although serious GI bleeding can occur without warning symptoms (e.g., hematemesis, melena, hematochezia), patients should be alert for these symptoms and should seek urgent medical care if any of these indicative symptoms occur (see WARNINGS, Serious Gastrointestinal Adverse Reactions ). In addition, patients should be alert for symptoms of ulcers (e.g., night time epigastric discomfort, vomiting, weight loss) and should seek medical attention if these symptoms occur. Patients who consume three or more alcoholic drinks every day should be counseled about the GI bleeding risks involved with the use of aspirin with alcohol.
- Patients should be informed of the symptoms of an anaphylactoid reaction or anaphylaxis (e.g., hives, difficulty breathing, swelling of face or throat). If these symptoms occur, patients should be instructed to seek immediate emergency help.
Codeine Phosphate:
- Since codeine phosphate may cause drowsiness and/or dizziness, patients should be advised to assess their individual response to codeine phosphate before engaging in potentially hazardous activities such as driving a motor vehicle or operating machinery (see WARNING, Sedation ).
- Patients should be advised to avoid alcoholic beverages while taking codeine phos-phate and to check with their doctor before taking other CNS depressants such as other opioids, benzodiazepines, tricyclic antidepressants, sedating antihistamines, or other sedatives (see WARNINGS, Respiratory Depression and Sedation ).
- Patients should be advised that codeine phosphate is a controlled substance. Codeine phosphate can result in psychological and physical dependence (see WARNING, De-pendence and Tolerance ).
- Codeine phosphate tablets should be placed in a secure place out of the reach of children.
- Patients should be advised that opioids, including codeine phosphate, can cause con-stipation and appropriate measures should be taken to reduce the risk of constipation (e.g., dietary changes, laxatives).
- Patients should be advised that opioids, including codeine phosphate, have been as-sociated with hypotension and gastrointestinal obstruction ( WARNINGS, Hypotension, Gastrointestinal Obstruction ).
- Patients should be advised that a subset of people who use codeine (ultra-rapid meta-bolizers) may convert codeine into its active metabolite, morphine, resulting that higher than expected exposure of morphine which can lead to increased opioid toxicity (see PRECAUTIONS, Ultra-rapid Metabolizers of Codeine ).
- Nursing mothers using codeine should be informed that a subset of people who use codeine (ultra-rapid metabolizers) may convert codeine into its active metabolite, mor-phine, resulting that higher than expected exposure of morphine which can lead to toxic serum levels of morphine in infants of nursing mothers. Nursing mothers should be informed how to recognize the symptoms of morphine toxicity in their infants, such as sedation, difficulty breastfeeding, breathing difficulties, and decreased tone (see PRE-CAUTIONS, Ultra-rapid Metabolizers of Codeine ).
Drug Interactions
Carisoprodol: The sedative effect of carisoprodol and other CNS depressants (e.g., alcohol, benzodiazepines, opioids, tricyclic antidepressants) may be additive. Therefore, caution should be exercised with patients who take more than one of these CNS depressants simultaneously. Concomitant use of carisoprodol and meprobamate, a metabolite of carisoprodol, is not recommended (see WARNINGS, Sedation ). Carisoprodol is metabolized in the liver by CYP2C19 to form meprobamate (see CLINICAL PHARMACOLOGY ). Co-administration of CYP2C19 inhibitors, such as omeprazole or fluvoxamine, with carisoprodol could result in increased exposure of carisoprodol and decreased exposure of meprobamate. Co-administration of CYP2C19 inducers, such as rifampin or St. John’s Wort, with carisoprodol could result in decreased exposure of carisoprodol and increased exposure of meprobamate. Low dose aspirin also showed an induction effect of CYP2C19. The full pharmacological impact of these potential alterations of exposures in terms of either efficacy or safety of carisoprodol is unknown.
Aspirin: Clinically important interactions may occur when certain drugs or alcohol are administered concomitantly with aspirin.
Alcohol: Concomitant use of aspirin with ≥ 3 alcoholic drinks may increase the risk of GI bleeding (see WARNINGS, Serious Gastrointestinal Adverse Reactions ).
Anticoagulants: Concomitant use of aspirin and anticoagulants (e.g., heparin, warfarin, clopidogrel) increase the risk of GI bleeding (see WARNINGS, Serious Gastrointestinal Adverse Reactions ). Additionally, aspirin can displace warfarin from protein binding sites, leading to prolongation of the international normalized ratio (INR).
Antihypertensives: The concomitant administration of aspirin with angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), beta¬-blockers, and diuretics may diminish the hypotensive effects of these anti-¬hypertensive products due to aspirin’s inhibition of renal prostaglandins, which may lead to decreased renal blood flow and increased sodium and fluid retention. Concomitant use of aspirin and acetazolamide can lead to high serum concentrations of acetazolamide due to competition at the renal tubule for secretion.
Corticosteroids: Concomitant administration of aspirin and corticosteroids may decrease salicylate plasma levels.
Methotrexate: Aspirin may enhance the toxicity of methotrexate due to displacement of methotrexate from its plasma protein binding sites and/or reduction of the renal clearance of methotrexate.
Nonsteroidal anti-inflammatory drugs (NSAIDs): The concurrent use of aspirin with selective and nonselective NSAIDs increases the risk of serious GI adverse reactions (see WARNINGS, Serious Gastrointestinal Adverse Reactions ).
Oral Hypoglycemics Agents: Aspirin may increase the serum glucose-lowering action of insulin and sulfonylureas leading to hypoglycemia.
Products that effect urinary pH: Ammonium chloride and other drugs that acidify the urine can elevate plasma salicylate concentrations. In contrast, antacids, by alkalinizing the urine, may decrease plasma salicylate concentrations.
Uricosuric Agents: Salicylates antagonize the uricosuric action of probenecid and sulfinpyrazone.
Codeine Phosphate:The sedative effects of codeine phosphate and other CNS depressants (e.g., alcohol, benzodiazepines, other opioids, tricyclic antidepressants) may be additive. Therefore, caution should be exercised with patients who take more than one of these CNS depressants simultaneously (see WARNINGS, Respiratory Depression and Sedation ).
Carcinogenesis, Mutagenesis, Impairment of Fertility:
No long-term studies of carcinogens have been done with Carisoprodol and Aspirin
Carisoprodol: Long term studies in animals have not been performed to evaluate the carcinogenic potential of carisoprodol.
Carisoprodol was not formally evaluated for genotoxicity. In published studies, carisoprodol was mutagenic in the in vitro mouse lymphoma cell assay in the absence of metabolizing enzymes, but was not mutagenic in the presence of metabolizing enzymes. Carisoprodol was clastogenic in the in vitro chromosomal aberration assay using Chinese hamster ovary cells with or without the presence of metabolizing enzymes. Other types of genotoxic tests resulted in negative findings. Carisoprodol was not mutagenic in the Ames reverse mutation assay using S. typhimurium strains with or without metabolizing enzymes, and was not clastogenic in an in vivo mouse micronucleus assay of circulating blood cells.
Carisoprodol was not formally evaluated for effects on fertility. Published reproductive studies of carisoprodol in mice found no alteration in fertility although an alteration in reproductive cycles characterized by a greater time spent in estrus was observed at a carisoprodol dose of 1200 mg/kg/day. In a 13¬-week toxicology study that did not determine fertility, mouse testes weight and sperm motility were reduced at a dose of 1200 mg/kg/day. In both studies, the no effect level was 750 mg/kg/day, corresponding to approximately 2.6 times the human equivalent dosage of 350 mg four times a day, based on a body surface area comparison.
The significance of these findings for human fertility is not known.
Aspirin: Administration of aspirin for 68 weeks in the feed of rats was not carcinogenic. In the Ames Salmonella assay, aspirin was not mutagenic; however, aspirin did induce chromosome aberrations in cultured human fibroblasts. Aspirin has been shown to inhibit ovulation in rats (see Pregnancy ).
Pregnancy: Pregnancy Category D.
It is not known whether Carisoprodol, Aspirin and Codeine Phosphate can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Adequate animal reproduction studies have not been conducted with Carisoprodol, Aspirin and Codeine Phosphate. Carisoprodol, Aspirin and Codeine Phosphate should be given to a pregnant woman only if clearly needed.
Carisoprodol: There are no data on the use of carisoprodol during human pregnancy. Animal studies indicate that carisoprodol crosses the placenta and results in adverse effects on fetal growth and postnatal survival. The primary metabolite of carisoprodol, meprobamate, is an approved anxiolytic. Retrospective, post¬marketing studies do not show a consistent association between maternal use of meprobamate and an increased risk for particular congenital malformations.
Teratogenic effects: Animal studies have not adequately evaluated the teratogenic effects of carisoprodol. There was no increase in the incidence of congenital malformations noted in the reproductive studies in rats, rabbits, and mice treated with meprobamate. Retrospective, post¬marketing studies of meprobamate during human pregnancy were equivocal for demonstrating an increased risk of congenital malformations following the first trimester exposure. Across studies that indicated an increased risk, the types of malformations were inconsistent.
Nonteratogenic effects: : In animal studies, carisoprodol reduced fetal weights, postnatal weight gain, and postnatal survival at maternal doses equivalent to 1 to 1.5 times the human dose (based on a body surface area comparison). Rats exposed to meprobamate in-¬utero showed behavioral alterations that persisted into adulthood. For children exposed to meprobamate in-¬utero, one study found no adverse effects on mental or motor development or IQ scores. Carisoprodol should be used during pregnancy only if the potential benefit justifies the risk to the fetus.
Aspirin:
Teratogenic effects:Prior to 30 weeks gestation, aspirin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Starting at 30 weeks gestation, aspirin should be avoided by pregnant women as premature closure of the fetal ductus arteriosus, which may result in fetal pulmonary hypertension and fetal death. Salicylate products have also been associated with alterations in maternal and neonatal hemostasis mechanisms, decreased birth weight, increased incidence of intracranial hemorrhage in premature infants, stillbirths, and neonatal death. Studies in rodents have show salicylates to be teratogenic when given in early gestation, and embryocidal when given in later gestation in doses considerably greater than usual therapeutic doses in humans.
Labor and Delivery
Carisoprodol: There is no information about the effects of carisoprodol on the mother and the fetus during labor and delivery.
Aspirin: Ingestion of aspirin within one week of delivery or during labor may prolong delivery or lead to excessive blood loss in the mother, fetus, or neonate. Prolonged labor due to prostaglandin inhibition has been reported with aspirin use.
Codeine Phosphate:The use of codeine phosphate during labor may lead to respiratory depression in the neonate.
Nursing Mothers
Carisoprodol: Very limited data in humans show that carisoprodol is present in breast milk and may reach concentrations two to four times the maternal plasma concentrations. In one case report, a breast-¬fed infant received about 4 to 6% of the maternal daily dose though breast milk and experienced no adverse effects. However, milk production was inadequate and the baby was supplemented with formula. In lactation studies in mice, female pup survival and pup weight at weaning was decreased. This information suggests that maternal use of carisoprodol may lead to reduced or less effective infant feeding (due to sedation) and/or decreased milk production. Caution should be exercised when carisoprodol is administered to a nursing woman.
Aspirin: Nursing mothers should avoid the use of aspirin because salicylate is excreted in breast milk, which may lead to bleeding in the infant.
Codeine Phosphate:Codeine is secreted into human milk. In women with normal codeine metabolism (normal CYP2D6 activity), the amount of codeine secreted into human milk is low. Despite the common use of codeine products to manage postpartum pain, reports of codeine-associated adverse reactions in nursing infants are rare. Nursing mothers who are ultra-rapid metabolizers of codeine have higher-than-expected levels of morphine (the active metabolite of codeine) in their blood, leading to higher levels of morphine in their breast milk and potentially dangerously high serum morphine levels in their breastfed infants. Therefore, in nursing mothers who are ultra-rapid metabolizers of codeine, the maternal use of codeine can lead to serious adverse reactions, including death; in their nursing infants and in the nursing mothers (see PRECAUTIONS, Ultra-rapid Metabolizers of Codeine ).
Prior to prescribing nursing mothers codeine phosphate, the risk of infant exposure to codeine and morphine through breast milk should be weighed against the benefits of breastfeeding for both the mother and the infant. If a codeine-containing product is selected, the lowest dose should be prescribed for the shortest period of time to achieve the desired clinical effect. Pre-scribers should closely monitor mother-infant pairs and notify treating pediatricians about the use of codeine during breastfeeding.
Pediatric Use: The efficacy, safety, and pharmacokinetics of Carisoprodol, Aspirin and Codeine Phosphate in pediatric patients less than 16 years of age have not been established.
Geriatric Use: The efficacy, safety, and pharmacokinetics of Carisoprodol, Aspirin and Codeine Phosphate in patients over 65 years of age have not been established.
CARISOPRODOL, ASPIRIN AND CODEINE PHOSPHATE ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact Mirror Pharmaceuticals at 1-862-210-8529 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
The following adverse reactions, which have occurred with the administration of the individual products alone may also occur with the use of Carisoprodol, Aspirin and Codeine Phosphate Tablets. The following events have been reported during post-approval individual use of carisoprodol and aspirin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Carisoprodol: The following events have been reported during post-¬approval use of carisoprodol. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular: Tachycardia, postural hypotension, and facial flushing (see OVERDOSAGE ).
Central Nervous System: Drowsiness, dizziness, vertigo, ataxia, tremor, agitation, irritability, headache, depressive reactions, syncope, insomnia, and seizures (see OVERDOSAGE ).
Gastrointestinal: Nausea, vomiting, and epigastric discomfort.
Hematologic: Leukopenia, pancytopenia.
Aspirin: TThe most common adverse reactions associated with the use of aspirin have been gastrointestinal, including abdominal pain, anorexia, nausea, vomiting, gastritis, and occult bleeding (see WARNINGS, Serious Gastrointestinal Adverse Reactions and PRECAUTIONS , Gastrointestinal Adverse Reactions). Other adverse reactions associated with the use of aspirin include elevated liver enzymes, rash, pruritus, purpura, intracranial hemorrhage, interstitial nephritis, acute renal failure, and tinnitus. Tinnitus may be a symptom of high serum salicylate levels (see OVERDOSAGE ).
Codeine Phosphate:Nausea, vomiting, constipation, miosis, sedation, dizziness.
DRUG ABUSE AND DEPEDENCE–Controlled Substance: Schedule C-III (see WARNINGS ).
Discontinuation of carisoprodol in animals or in humans after chronic administration can produce withdrawal signs, and there are published case reports of human carisoprodol dependence.
In vitro studies demonstrate that carisoprodol elicits barbiturate-like effects. Animal behavioral studies indicate that carisoprodol produces rewarding effects. Monkeys self-administer carisoprodol. Drug discrimination studies using rats indicate that carisoprodol has positive reinforcing and discriminating effects similar to barbital, meprobamate, and chlordiazepoxide.
OVERDOSAGE
Signs and Symptoms: Any of the following signs and symptoms which have been reported with overdose of the individual products may occur with overdose of Carisoprodol and Aspirin and may be modified to a varying degree by the effects of the other products present in Carisoprodol and Aspirin.
Carisoprodol: Overdosage of Carisoprodol commonly produces CNS depression. Death, coma, respiratory depression, hypotension, seizures, delirium, hallucinations, dystonic reactions, nystagmus, blurred vision, mydriasis, euphoria, muscular incoordination, rigidity, and/or headache have been reported with Carisoprodol overdosage. Many of the Carisoprodol overdoses have occurred in the setting of multiple drug overdoses (including drugs of abuse, illegal drugs, and alcohol). The effects of an overdose of carisoprodol and other CNS depressants (e.g., alcohol, benzodiazepines, opioids, tricyclic antidepressants) can be additive even when one of the drugs has been taken in the recommended dosage. Fatal accidental and non-¬accidental overdoses of Carisoprodol have been reported alone or in combination with CNS depressants.
Aspirin: Salicylate toxicity may result from an overdose of an acute ingestion or chronic intoxication. Mild to moderate salicylate poisoning is usually associated with plasma salicylic concentrations about 200 µg/mL and is characterized by tinnitus, hearing difficulty, headache, dim vision, dizziness, tachypnea, increased thirst, nausea, vomiting, sweating, and diarrhea. In the early stages of overdose, CNS stimulation and respiratory alkalosis can occur; however, in the later stages CNS depression and metabolic acidosis can occur.
Symptoms and signs of severe salicylate poisoning, associated with plasma salicylic concentrations greater that 400 g/mL, include hyperthermia, dehydration, delirium, GI hemorrhage, pulmonary edema, and CNS depression (e.g., coma). Death is usually due to respiratory failure or cardiovascular collapse.
Overdose of aspirin in pediatric patients: Salicylate poisoning should be considered in pediatric patients with symptoms of vomiting, hyperpnea, and hyperthermia. Salicylate poisoning should be considered in infants with metabolic acidosis and all pediatric patients with severe salicylate poisoning.
Codeine Phosphate:Acute overdose of opioids, including codeine phosphate, is characterized by CNS depression (somnolence progressing to coma), respiratory depression, hypotension, miosis, skeletal muscle flaccidity, and cold and clammy skin.
Treatment of Overdosage: Provide symptomatic and supportive treatment, as indicated. For more information on the management of an overdose of Carisoprodol and Aspirin tablets, USP contact a Poison Control Center.
Carisoprodol:Basic life support measures should be instituted as dictated by the clinical presentation of the carisoprodol overdose. Induced emesis is not recommended due to the risk of CNS and respiratory depression, which may increase the risk of aspiration pneumonia. Gastric lavage should be considered soon after ingestion (within one hour). Circulatory support should be administered with volume infusion and vasopressor agents if needed. Seizures should be treated with intravenous benzodiazepines and the reoccurrence of seizures may be treated with phenobarbital. In cases of severe CNS depression, airway protective reflexes may be compromised and tracheal intubation should be considered for airway protection and respiratory support. The following types of treatment have been used successfully with an overdose of meprobamate, a metabolite of carisoprodol: activated charcoal (oral or via nasogastric tube), forced diuresis, peritoneal dialysis, and hemodialysis (carisoprodol is also dialyzable). Careful monitoring of urinary output is necessary and overhydration should be avoided. Observe for possible relapse due to incomplete gastric emptying and delayed absorption.
Aspirin: Since there are no specific antidotes for salicylate poisoning, the aim of the treatment is to enhance elimination of salicylate; reduce further salicylate absorption; correct fluid, electrolyte, or acid/base imbalances; and provide cardio‑respiratory support. The acid base status should be followed closely with serial serum pH determinations (using arterial blood gas). If acidosis is present, intravenous sodium bicarbonate should be given, along with adequate hydration, until salicylate levels decrease to within the therapeutic range. To enhance elimination, forced diuresis and alkalinization of the urine may be beneficial. Gastric emptying and/or lavage are recommended as soon as possible after ingestion, even if the patient has vomited spontaneously. After lavage and/or emesis, administration of activated charcoal is beneficial, if less than 3 hours have passed since ingestion. Charcoal absorption should not be employed prior to emesis and lavage. In patients with renal insufficiency or in cases of life-threatening aspirin intoxication, hemodialysis or peritoneal dialysis is usually required.
Additional treatment of aspirin overdose in pediatric patients: Pediatric patients should be sponged with tepid water. Infusion of glucose may be required to control hypoglycemia. Exchange transfusion may be indicated in infants and young children.
Codeine Phosphate:After a severe opioid overdose, primary attention should be given to the need for re-establishment of a patent airway and institution of assisted ventilation. Elimination or evacuation of gastric contents may be necessary in order to eliminate unabsorbed drug. Before attempting treatment by gastric emptying or activated charcoal, care should be taken to secure the airway. Pure opioid antagonist (e.g., naloxone, nalmefene) are specific antidotes to severe respiratory and CNS depression resulting from opioid overdose. If the response to these opioid antagonists is sub-optimal, additional antagonist should be administered. Since the duration of action of codeine may exceed that of the opioid antagonist, the patient’s respiratory status should be continuously monitored for the need for additional doses of antagonist to maintain adequate respiration.
CARISOPRODOL, ASPIRIN AND CODEINE PHOSPHATE DOSAGE AND ADMINISTRATION
The recommended dose of Carisoprodol, Aspirin and Codeine Phosphate tablets, is 1 or 2 tablets, four times daily in adults. One Carisoprodol, Aspirin and Codeine Phosphate tablet contains 200 mg of Carisoprodol, 325 mg of Aspirin and 16 mg of Codeine Phosphate. The maximum daily dose (i.e., two tablets taken four times daily) will provide 1600 mg of carisoprodol, 2600 mg of aspirin and 128 mg of codeine phosphate per day. The recommended maximum duration of Carisoprodol, Aspirin and Codeine Phosphate tablets use is up to two or three weeks.
HOW SUPPLIED
Carisoprodol, Aspirin and Codeine Phosphate Tablets, USP 200 mg/ 325 mg/ 16 mg are yellow and white color, round unscored convex, two layered tablets debossed on yellow layer with “CL” over “024” and plain on the white layer. The tablets are available in:
Bottles of 100, NDC 52682-024-01.
Bottles of 500, NDC 52682-024-03.
Storage: Store at controlled room temperature 20° - 25°C (68° - 77°F). Protect from moisture.
Dispense in a tight container.
To report SUSPECTED ADVERSE REACTIONS, Mirror Pharmaceuticals at 1-862-210-8529 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch.
Manufactured by:
Mirror Pharmaceuticals, LLC
Fairfield, NJ 07004 USA
5511


Carisoprodol, Aspirin and Codeine PhosphateCarisoprodol, Aspirin and Codeine Phosphate TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||