Camphor, Menthol
DRUG FACTS
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients (% by weight)
- Purpose
- Camphor, Menthol Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- Questions?
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active Ingredients (% by weight)
Camphor 4.0%
Menthol 10.0%
Purpose
Analgesic (pain relief)
Camphor, Menthol Uses
For the temporary relief of minor aches and pains of muscles and joints associated with simple backache, arthritis, strains, bruises and sprains, etc.
Warnings
- For external use only.
- Caution: Discontinue use if excessive irritation of the skin develops. Avoid getting into eyes or on mucous membranes.
- If the conditions worsens, or if the symptoms persists for more than 7 days or clears up and occurs again within a few days, discontinue use of this product and consult a doctor.
- Do not apply to wounds or damaged skin. Do not bandage tightly.
OTC - Keep Out of Reach of Children Section
Keep out of reach of children.
Other Warnings
- Use only as directed.
- Do not use if pregnant or breastfeeding.
- If swallowed, get medical help or contact a poison Control center right away.
Directions
Adults and children12 years of age or older: Using the roll on applicator massage a liberal amount of gel directly on the affected area not more than 3 or 4 times daily.
Children under the age of 12: Do not use, consult a doctor.
Other Information
Store under normal storage conditions. Store away from Children.
Inactive Ingredients
Acrylates copolymer, boswellia, chondroitin sulphate, denatured alcohol, eucalyptus oil, glucosamine sulphate, glycerin,ilex, magnesium chloride, methylsulfonylmethane (MSM), peppermint oil, propylene glycol,triethanolamine, water.
Questions?
416-967-3746
Principal Display Panel
PROFESSIONAL THERAPY EXTRA STRENGTHEXTRA FUERTE
MuscleCare TM
PAIN RELIEVING GEL/ GEL ALIVIANTE DEL DOLOR
by/ por el Dr. Chris Oswald
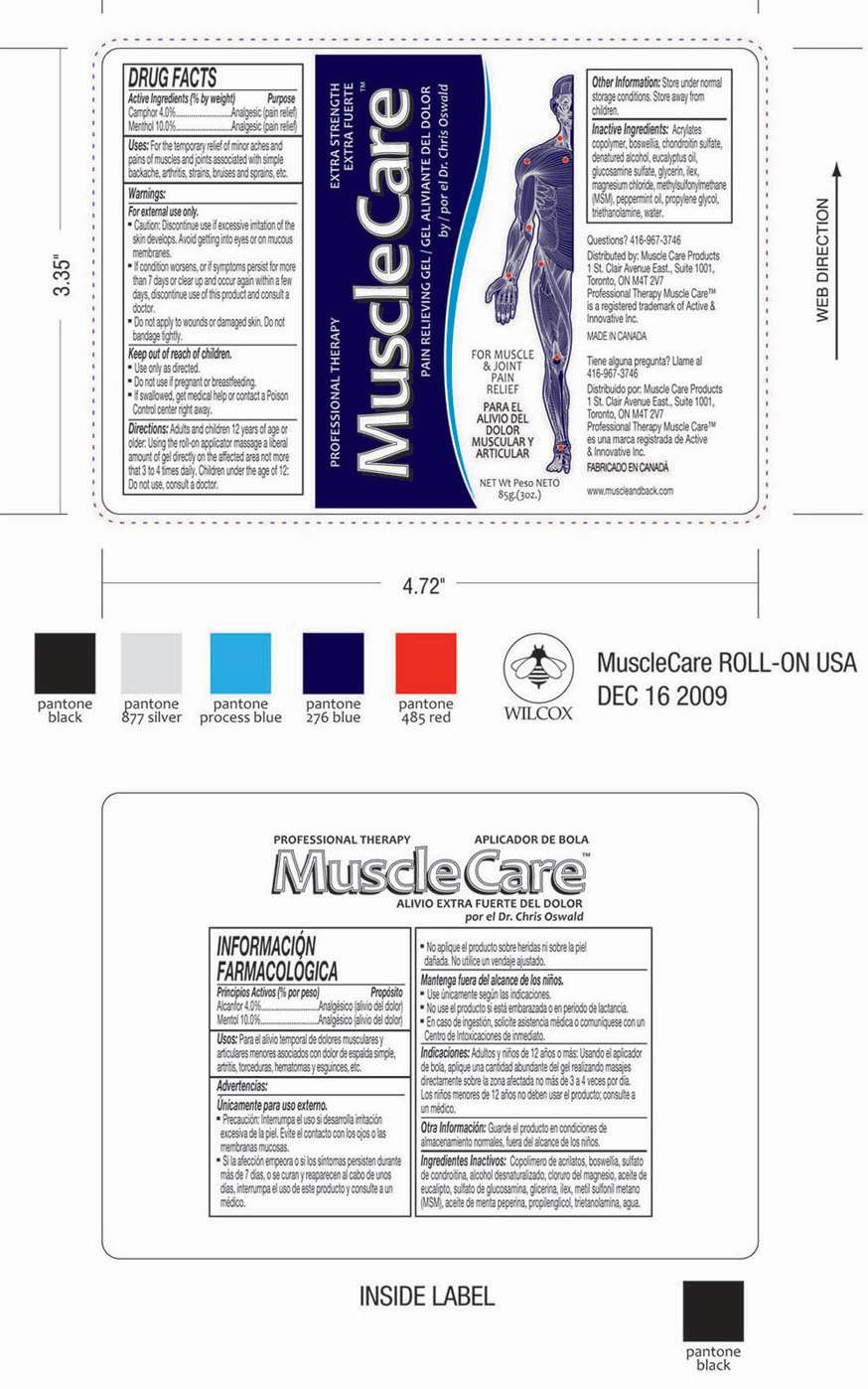
Camphor, MentholCamphor, Menthol GEL
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||