Borba Age Defying
BORBA AGE DEFYING DAILY HYDRATOR SPF 30+
FULL PRESCRIBING INFORMATION: CONTENTS*
- Borba Age Defying Uses
- Warnings
- Directions
- Inactive Ingredients
- Questions or Comments?
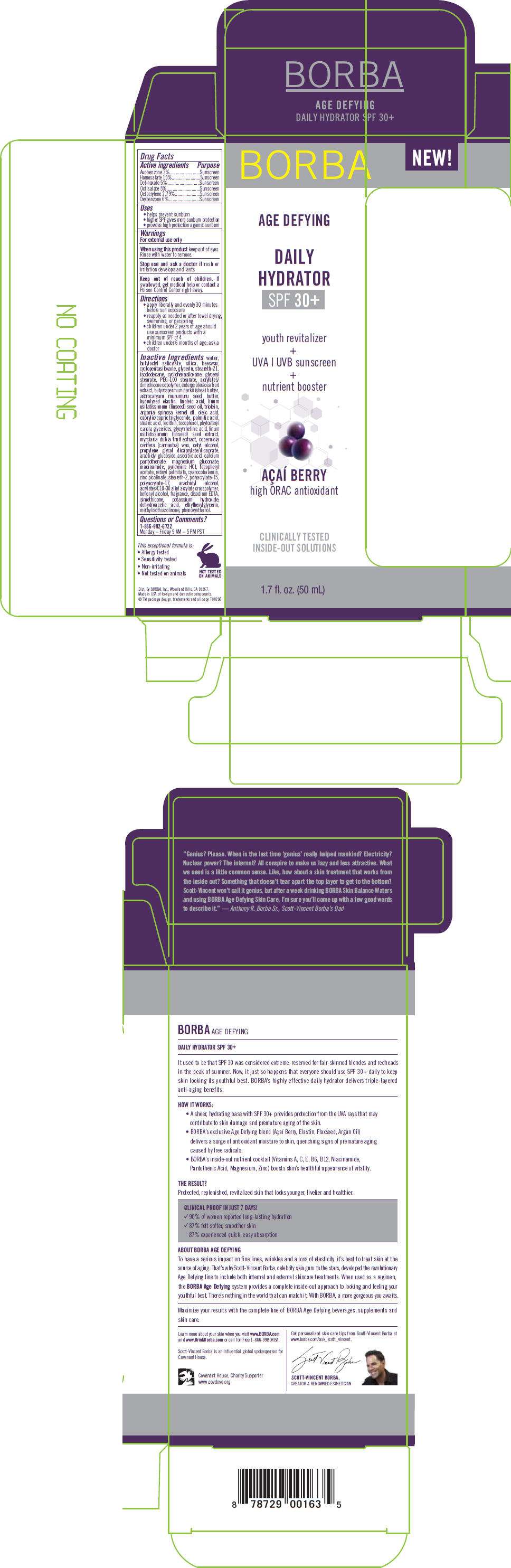
- PRINCIPAL DISPLAY PANEL - 50 mL Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Purpose
| Active ingredients | Purpose |
|---|---|
| Avobenzone 3% | Sunscreen |
| Homosalate 10% | Sunscreen |
| Octinoxate 5% | Sunscreen |
| Octisalate 5% | Sunscreen |
| Octocrylene 2.79% | Sunscreen |
| Oxybenzone 6% | Sunscreen |
Borba Age Defying Uses
- helps prevent sunburn
- higher SPF gives more sunburn protection
- provides high protection against sunburn
Warnings
For external use only
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash or irritation develops and lasts
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply liberally and evenly 30 minutes before sun exposure
- reapply as needed or after towel drying, swimming, or perspiring
- children under 2 years of age should use sunscreen products with a minimum SPF of 4
- children under 6 months of age: ask a doctor
Inactive Ingredients
water, butyloctyl salicylate, silica, beeswax, cyclopentasiloxane, glycerin, steareth-21, isododecane, cyclohexasiloxane, glyceryl stearate, PEG-100 stearate, acrylates/dimethicone copolymer, euterpe oleracea fruit extract, butyrospermum parkii (shea) butter, astrocaryum murumuru seed butter, hydrolyzed elastin, linoleic acid, linum usitatissimum (linseed) seed oil, triolein, argania spinosa kernel oil, oleic acid, caprylic/capric triglyceride, palmitic acid, stearic acid, lecithin, tocopherol, phytosteryl canola glycerides, glycyrrhetinic acid, linum usitatissimum (linseed) seed extract, myrciaria dubia fruit extract, copernicia cerifera (carnauba) wax, cetyl alcohol, propylene glycol dicaprylate/dicaprate, arachidyl glucoside, ascorbic acid, calcium pantothenate, magnesium gluconate, niacinamide, pyridoxine HCl, tocopheryl acetate, retinyl palmitate, cyanocobalamin, zinc picolinate, steareth-2, polyacrylate-15, polyacrylate-17, arachidyl alcohol, acrylates/C10-30 alkyl acrylate crosspolymer, behenyl alcohol, fragrance, disodium EDTA, simethicone, potassium hydroxide, dehydroacetic acid, ethylhexylglycerin, methylisothiazolinone, phenoxyethanol.
Questions or Comments?
1-866-992-6722
Monday – Friday 9 AM – 5 PM PST
Dist. By BORBA, Inc., Woodland Hills, CA 91367.
PRINCIPAL DISPLAY PANEL - 50 mL Carton
NEW!
BORBA
AGE DEFYING
DAILY
HYDRATOR
SPF 30+
youth revitalizer
+
UVA | UVB sunscreen
+
nutrient booster
AÇAÍ BERRY
high ORAC antioxidant
CLINICALLY TESTED
INSIDE-OUT SOLUTIONS
1.7 fl. oz. (50 mL)