Bio-Scriptives Extreme AF
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Purpose
- Bio-Scriptives Extreme AF Uses
- Warnings
- Stop use and ask a doctor if
- Directions
- Inactive Ingredients
- Reference
- Labeling
- Keep out of reach of children
FULL PRESCRIBING INFORMATION
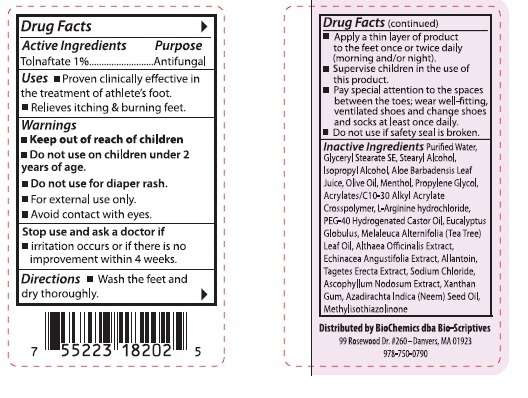
Active Ingredients
Tolnaftate 1%
Purpose
Antifungal
Bio-Scriptives Extreme AF Uses
Warnings
Stop use and ask a doctor if
- irritation occurs or if there is no improvement within 4 weeks.
Directions
Inactive Ingredients
Purified Water, Glyceryl Stearate SE, Stearyl Alcohol, Isopropyl Alcohol, Aloe Barbadensis Leaf Juice, Olive Oil, Menthol, Propylene Glycol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, L-Arginine hydroxide, PEG-40 Hdrogenated Castor Oil, Eucalyptus Globulus, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Althaea Officinalis Extract, Echinacea Angustifolia Extract, Allantoin, Tagetes Erecta Extract, Sodium Chloride, Ascophyllum Nodosum Extract, Xanthan Gum, Azadirachta Indica (Neem) Seed Oil, Methylisothiazolinone
Reference
Distributed by BioChemics dba Bio-Scriptives
99 Rosewood Dr. #260 - Danvers, MA 01923
978-750-0790
Labeling

Keep out of reach of children
Enter section text here
Bio-Scriptives Extreme AFTolnaftate CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||