Axe Clinical Protection
Front and Back PDP Axe Clinical Protection AP and Deod., PDP, outer carton and drug facts
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredient Purpose
Aluminum Zirconium Tetrachlorohydrex GLY (20%).................anti-perspirant
Warnings For External Use only
Questions? Call toll-free 1-800-450-7580
Do not useAsk a doctor before use if you have kidney disease
Stop use if rash or irritation occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.

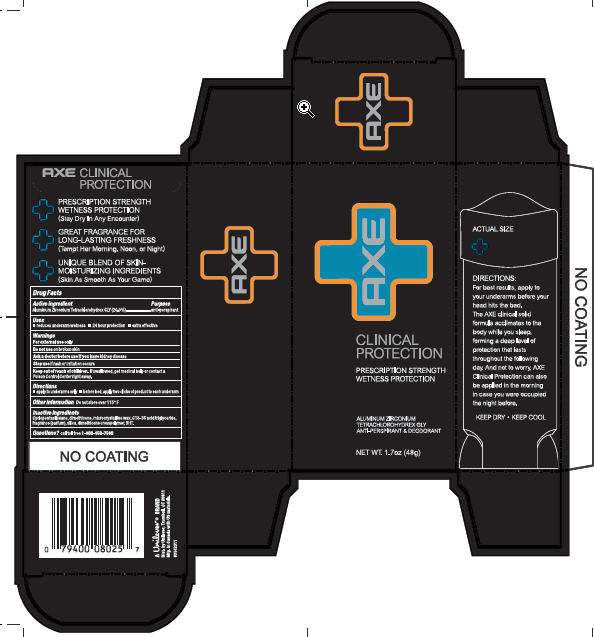
Axe Clinical ProtectionAluminum Zirconium Tetrachlorohydrex GLY STICK
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!