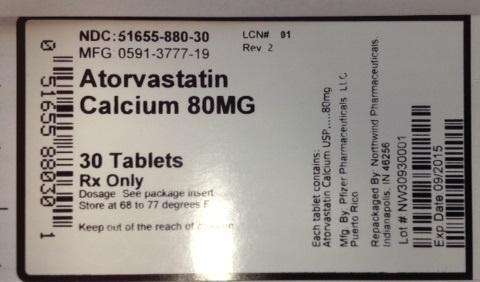
Atorvastatin Calcium
Northwind Pharmaceuticals
Northwind Pharmaceuticals
FULL PRESCRIBING INFORMATION
NDC:51655-880-30
MFG: 0591-3777-19
Atorvastatin Calcium 80 mg
30 tablets
RX only
Dosage: See package insert
Store at 68 to 77 degrees F
Keep out of the reach of children.
Each tablet contains: Atorvastatin Calcium USP 80mg
Mfg By: Pfizer Pharmaceuticals, Puerto Rico
Repackaged by Northwind Pharmaceuticals Indianapolis, IN 46256
Lot # NW30930001
Exp Date: 09/2015
Atorvastatin CalciumAtorvastatin Calcium TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!