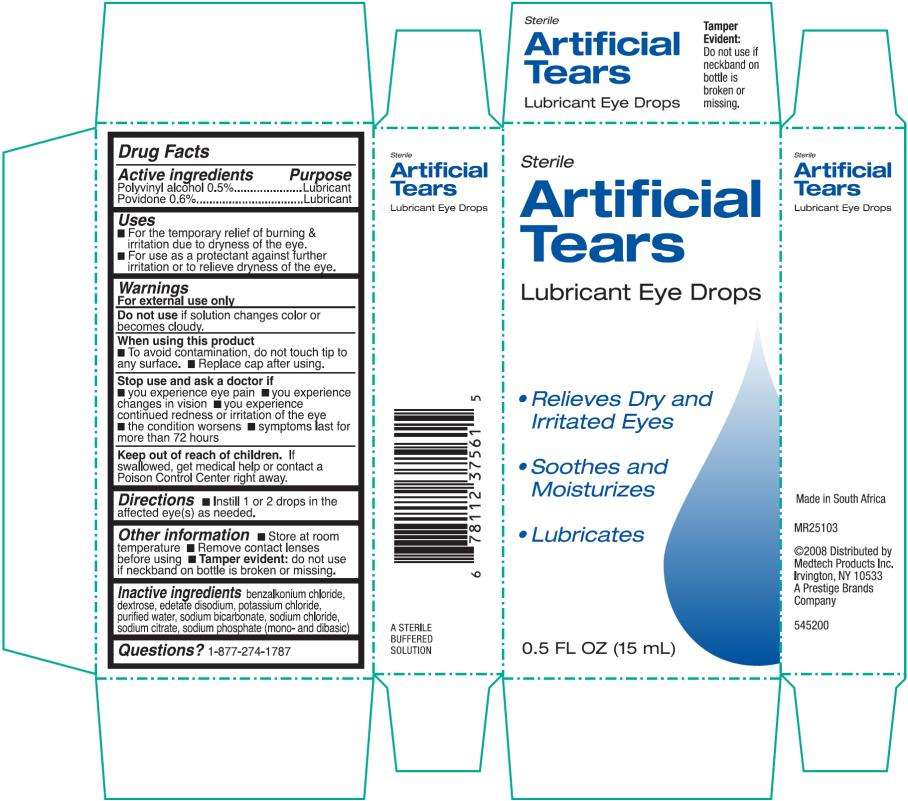
Artificial Tears Lubricant Eye
Prestige Brands Holdings, Inc.
Artificial Tears Lubricant Eye Drops
FULL PRESCRIBING INFORMATION
Drug Facts
Polyvinyl alcohol 0.5%
Povidone 0.6%
Lubricant
- For the temporary relief of burning & irritations due to dryness of the eye.
- For use as a protectant against further irritation or to relieve dryness of the eye.
For external use only
solution changes color or becomes cloudy.
- to avoid contamination, do not touch tip to any surface.
- replace cap after using.
- you experience eye pain
- you experience changes in vision
- you experience continued redness or irritation of the eye
- the condition worsens
- symptoms last for more than 72 hours
If swallowed, get medical help or contact a Poison Control Center right away.
- Instill 1 to 2 drops in the affected eye(s) as needed.
- Store at room temperature
- Remove contact lenses before using
- Tamper Evident: do not use if neckband on bottle is broken or missing.
benzalkonium chloride, dextrose, edetate disodium, potassium chloride, purified water, sodium bicarbonate, sodium chloride, sodium citrate, sodium phosphate (mono- and dibasic)
1-877-274-1787
Sterile
Artificial Tears Lubricant Eye Drops
0.5 FL OZ (15 mL)
Artificial Tears Lubricant EyePolyvinyl Alcohol and Povidone LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!